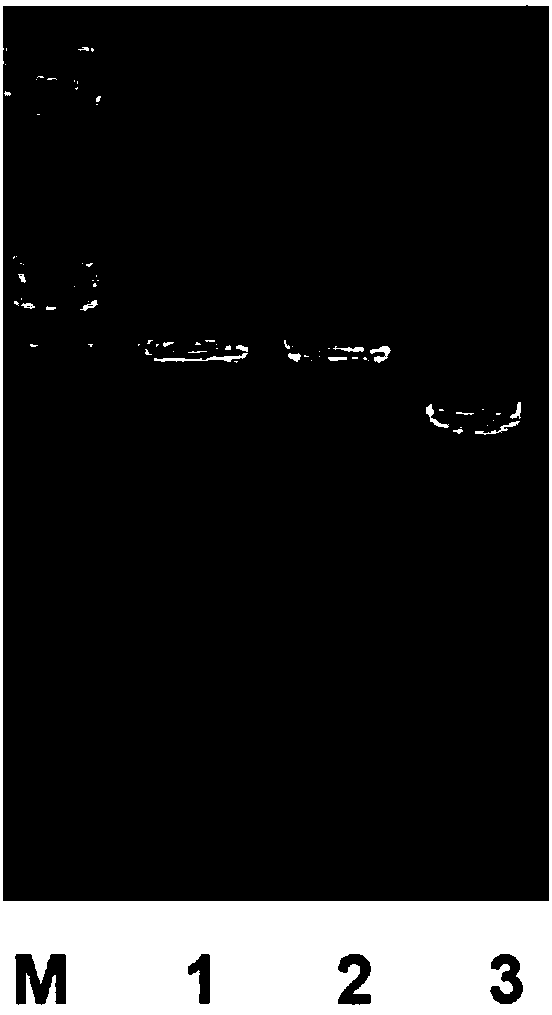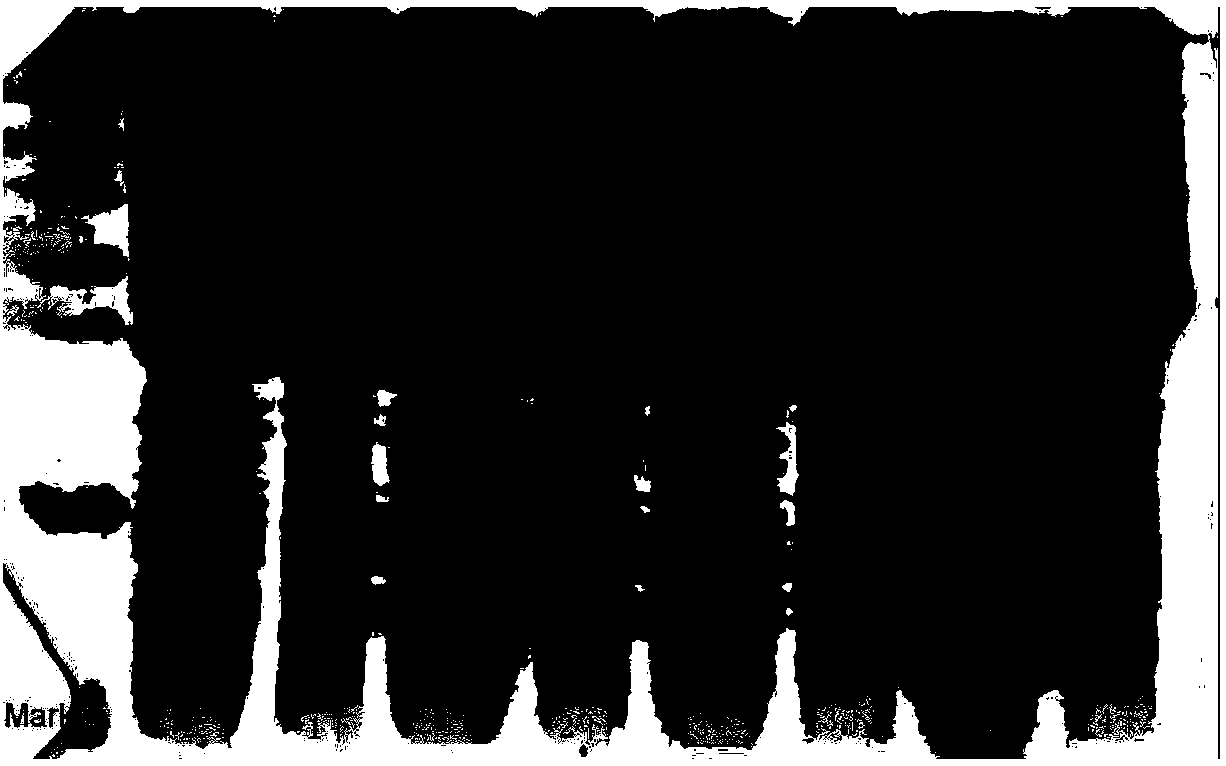Radioactive anti-peptide mutant protein and preparation and purification method thereof
A technology for mutant proteins and purification methods, which is applied in the fields of peptide preparation methods, chemical instruments and methods, and botanical equipment and methods, can solve the problems of insufficient consideration of the structural integrity and protein folding properties of flagellin proteins, and achieve easy Preservation, improving purity, reducing the effect of degradation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Preparation and purification of radioresistant peptide muteins
[0030] 1) Construction of radioresistant peptide mutein expression vector
[0031] Synthesize the cDNA of the radioresistant peptide mutein according to the nucleotide sequence shown in SEQ ID NO: 2 by base synthesis method, and connect it to the pBV220 temperature-inducible expression vector after restriction enzyme digestion to construct the pBV220-FKT recombinant expression vector , see the results of vector digestion and identification figure 1 , and then use this vector to transform Escherichia coli JM109, screen positive clones on ampicillin-resistant LB medium, and establish a seed bank of engineering bacteria.
[0032] 2) Fermentation and electrophoresis identification of radioresistant peptide mutant protein
[0033] The JM109 engineered bacteria containing the recombinant bacterial flagellin-derived polypeptide radioresistant peptide mutant carrier were shaken and cultured overnight a...
Embodiment 2
[0048] Example 2 Preparation and Purification of Unmutated Radioresistant Peptide Protein CBLB502
[0049] The preparation method of the radioresistant peptide protein CBLB502 is similar to Example 1, the difference is that when constructing the expression vector, the cDNA of the radioresistant peptide protein CBLB502 is synthesized in vitro by base synthesis method, and connected to the temperature-inducible expression vector In pBV-220; then the JM109 engineering bacteria containing the recombinant bacterial flagellin-derived polypeptide radioantipeptide protein CBLB502 carrier were fermented, expressed and purified according to the conditions of step 2)-step 6), and electrophoresis analysis was carried out, and the results showed that the present invention The method is also applicable to the preparation and purification of unmutated radioresistant peptide protein ( Figure 9 ).
Embodiment 3
[0050] Example 3 Biological Activity Assay of Radioresistant Peptide Mutant Protein
[0051] Select the positive clone with the best response to NF-kB stimulating activity to inoculate a 96-well plate, the inoculation medium is DMEM containing only 10% fetal bovine serum without hygromycin B, and the number of cells per well is 1×10 4 , a total of 8 groups, each with 3 replicate wells. After 24 hours, the radioresistant peptide mutant protein was stimulated, and the administration concentration was 10 -12 ~10 -5 g / ml, after 6 hours, detect the value of the luciferase reporter gene ( Figure 10 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com