Preparation method and applications of ganglioside derivatives containing unsaturated fatty acid chains
An unsaturated fatty acid and ganglioside technology, applied in the biological field, can solve the problem of no systematic research on ganglioside derivatives, affecting the neurite outgrowth activity of gangliosides, and affecting the biological activity of gangliosides. and other problems, to achieve the effect of shortening purification steps and purification time, avoiding cleaning steps, and being easy to remove
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
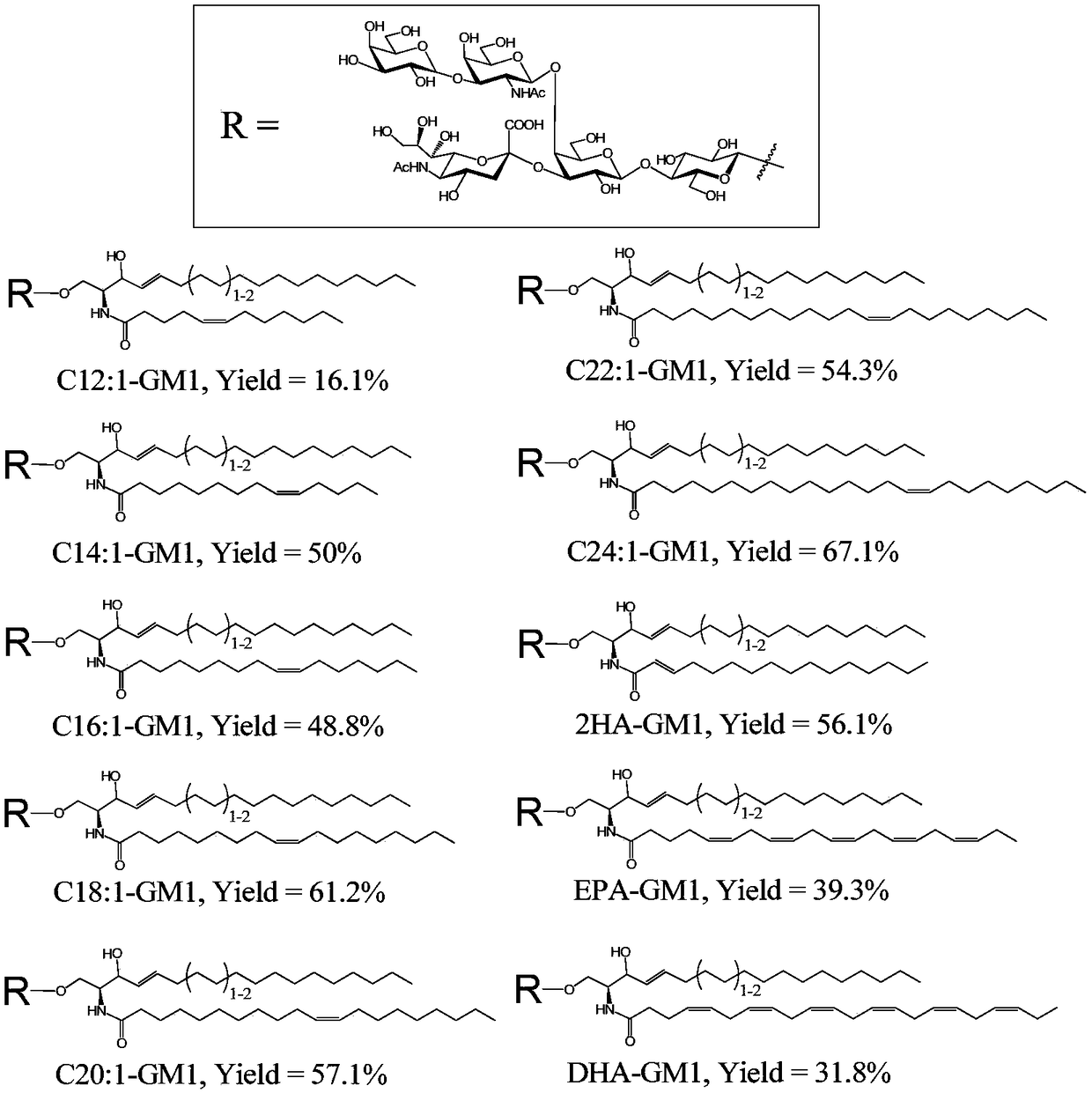
[0067] Example 1. Enzymatic synthesis of a library of ganglioside derivatives with unsaturated fatty acid chains
specific Embodiment approach
[0068] Since the ganglioside GM1 has the most clear effect on promoting neurite growth, we first established a derivative library of GM1 with unsaturated fatty acid chains, and the specific implementation method is as follows:
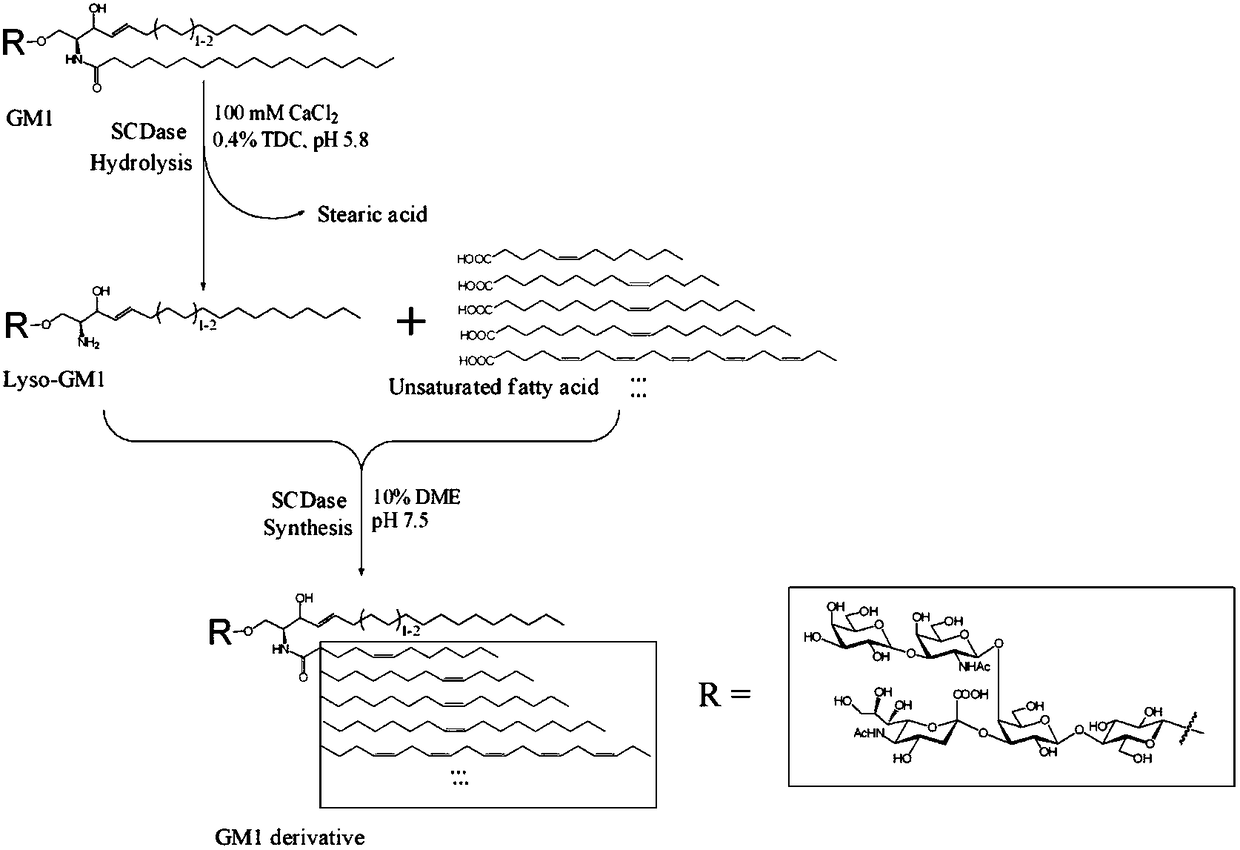
[0069] For enzymatic synthesis of GM1 derivatives containing unsaturated fatty acid chains, see figure 2 .
[0070] First, prepare the sphingolipid ceramide N-deacylase required for the catalytic synthesis reaction and the substrate lysoganglioside GM1 (Lyso-GM1) required for the synthesis of GM1 derivatives. The specific steps follow our previous application Patent (application number: CN201410508221.X) and published articles (J Lipid Res, 2015, 56:1836-1842) for preparation.
[0071] Synthetic reaction system: the reaction system contains about 10% ethylene glycol dimethyl ether (DME), about 20 mg of lyso-GM1, and 1.5 mM unsaturated fatty acid in 20 ml of 50 mM Tris-HCl (pH 7.5). To the reaction system, 1ml of 1mg / ml SCDase was added every 8-12h f...
Embodiment 2
[0076] Example 2: Separation and purification of a library of ganglioside derivatives with unsaturated fatty acid chains
[0077] After the catalytic synthesis reaction is completed, it is necessary to separate and purify the derivatives with unsaturated fatty acid chain gangliosides from the reaction mixture. Sep-Pak tC18 solid-phase extraction column (one 500mg column, one 5g column) of Waters Company was used for separation and purification. Since it does not contain surfactant, the synthesis reaction solution can be purified directly on the solid phase extraction column: first, the reaction mixture flows through a small Sep-Pak tC18 solid phase extraction column (500mg), and then the flow-through solution flows through a A large Sep-Pak tC18 solid-phase extraction cartridge (5g), washed 50ml with water, then 50ml with 50% methanol, and finally washed the derivative with 100% methanol, concentrated by rotary evaporation and lyophilized. In addition, the reaction mixture ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com