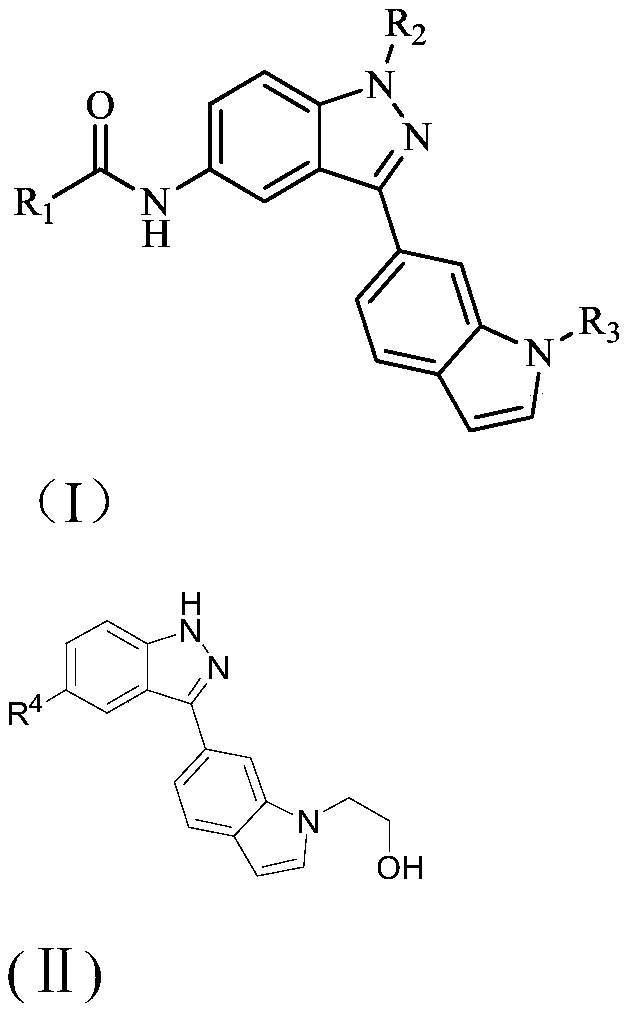
A kind of 3-(indol-5-yl)-indazole derivative and its application
A derivative, indazole technology, applied in the field of 3--indazole derivatives and its application, can solve the problems of poor water solubility and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0026] The synthesis of embodiment 13-iodo-5-nitro-1H-indazole
[0027] Dissolve 5-nitroindazole (5g, 30.8mmol) in 30mL N, N-dimethylformamide, add iodine (15.66g, 61.6mmol) and potassium hydroxide solid (3.58g, 63.7mmol) in sequence, React at 65°C for 3h. After the reaction, 50 mL of 1M HCl and 80 mL of 10% sodium thiosulfate solution were added, and after stirring for 30 min, ice water was added to the reaction to precipitate a yellow bottom, filtered with suction, and the filter cake was washed with dichloromethane. The filter cake was further recrystallized from ethyl acetate to obtain 7.99 g of the yellow target product, with a yield of 90%.
Embodiment 2
[0028] Example 2 3-iodo-5-nitro-1-((2-(trimethylsilyl)ethoxy)methyl)-1H-indazole (9a)
[0029] Add sodium hydride (60%; 720mg, 17.99mmol) to 3-iodo-5-nitro-1H-indazole (2g, 6.92mmol) solution in 15mL N,N-dimethylformamide at 0°C, and add after 30min 2-(Trimethylsilyl)ethoxymethyl chloride (1.50mL, 8.30mmol), stirred at room temperature for 3h. After the reaction was completed, it was quenched by adding ice water, extracted by adding ethyl acetate, washed with saturated sodium chloride solution, dried over anhydrous magnesium sulfate, and the solvent was removed by rotary evaporation. The crude product was purified by column chromatography to obtain 2.6 g of product with a yield of 90%.
Embodiment 3
[0030] Example 3 Synthesis of 1-benzyl-3-iodo-5-nitro-1H-indazole (9b)
[0031] 3-iodo-5-nitro-1H-indazole (400mg, 1.38mmol) was dissolved in 10mL acetone, potassium carbonate (382mg, 2.76mmol) and benzyl bromide (164μL, 1.38mmol) were added, and the reaction was heated under reflux for 2h. After the reaction was completed, quenched by adding saturated ammonium chloride, the acetone solvent was removed by rotary evaporation, extracted by adding ethyl acetate, washed with saturated sodium chloride solution, dried over anhydrous magnesium sulfate, and the solvent was removed by rotary evaporation, and the crude product was purified by column chromatography to obtain 497.5 mg product, yield 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com