A method for solvothermally assisted preparation of multi-phase titanate red long-lasting phosphor
A long-lasting phosphor and multi-phase titanate technology, applied in chemical instruments and methods, luminescent materials, climate sustainability, etc., can solve problems such as difficult ionization, achieve simple process, low pollution, and avoid easy loss of activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0020] Example 1 [Ca 0.8 Zn 0.2 TiO 3 ]
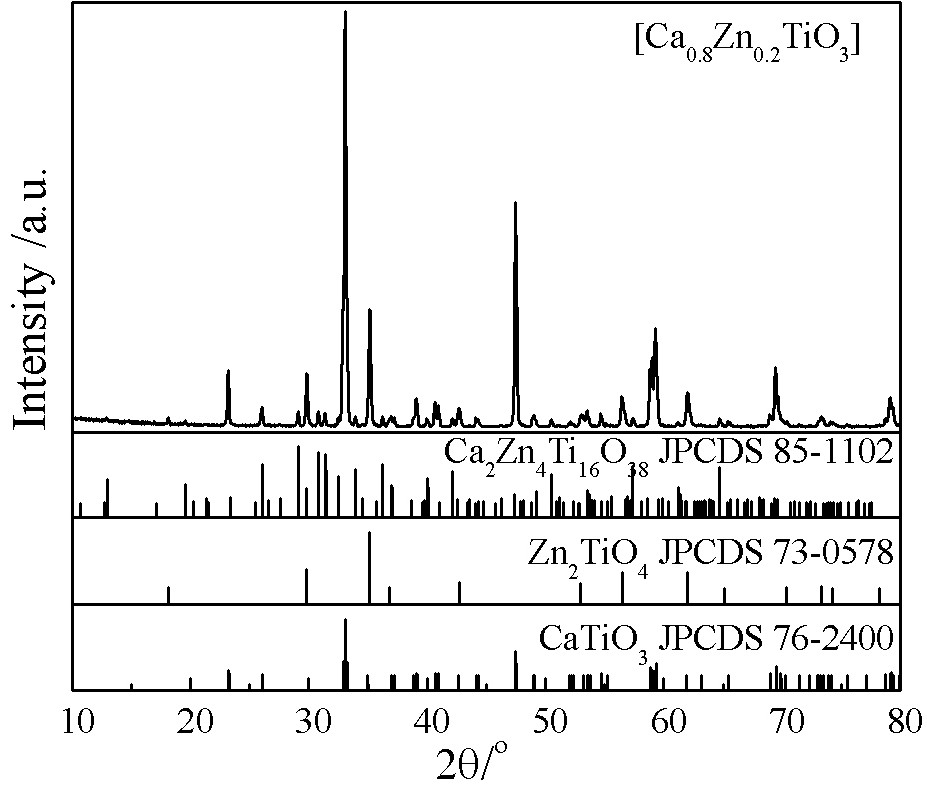
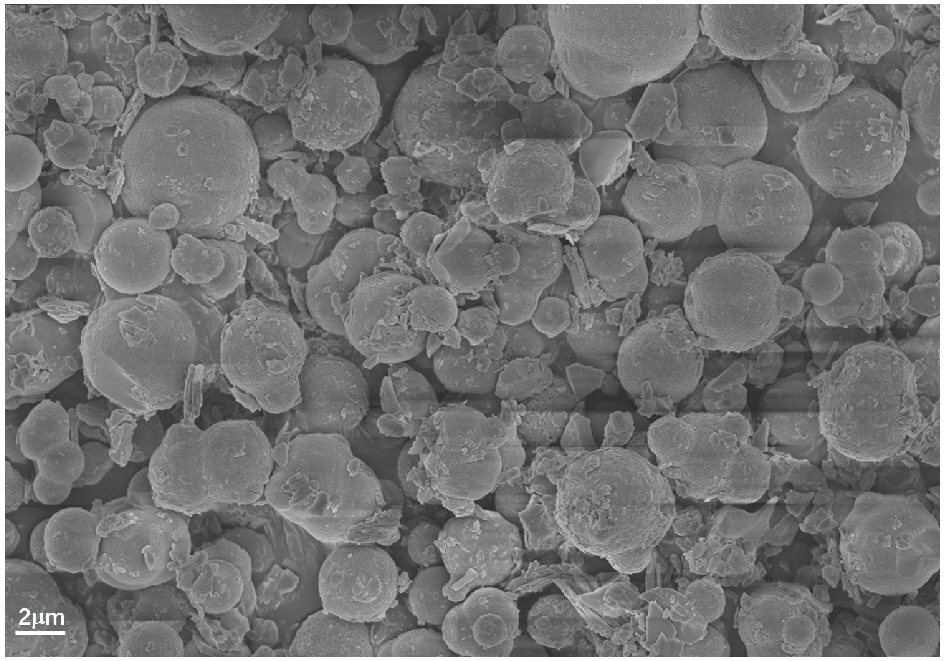
[0021] Weigh 2.5527g (about 5mL) of butyl titanate, dissolve it in 75 mL of n-butanol, stir for 30 min under a magnetic stirrer at 300 r / min, add 2 mL of 3mol / L calcium nitrate, 0.5 mL of 3mol / L calcium nitrate dropwise / L zinc nitrate solution, stirred for 1 h, transferred to a high-pressure reactor to maintain a filling degree of 80%, sealed and reacted at 200 °C for 2 h, and after the reactor was cooled to room temperature, the mixed suspension of the precursor was mixed at 130 After drying at ℃, the precursor powder was ground and dried and calcined at 1100 ℃ for 5 h. get [Ca 0.8 Zn 0.2 TiO 3 ]’s multiphase titanate, its phase composition is as follows figure 1 As shown, the microscopic morphology is as figure 2 shown.
example 2
[0022] Example 2 [Ca 0.8 Zn 0.2 TiO 3 ]: Pr 3+
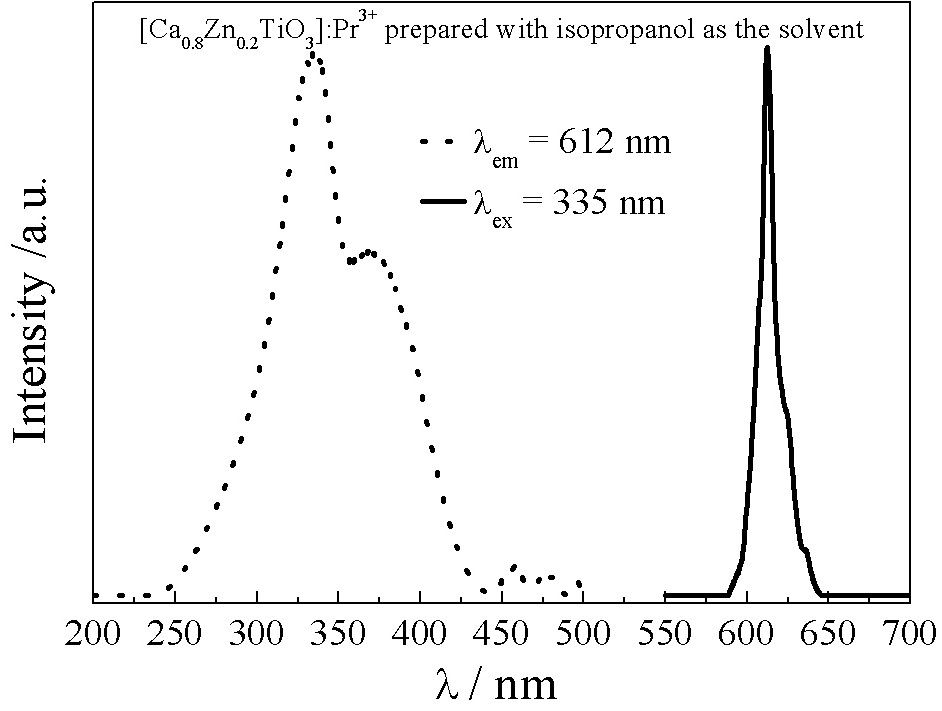
[0023] The preparation method is the same as Example 1, except that butyl titanate is dissolved in 25 mL of isopropanol, and 0.75 mL of 0.0005 mol / L praseodymium nitrate solution is added to obtain [Ca 0.8 Zn 0.2 TiO 3 ]: Pr 3+ The complex phase titanate red phosphor, its excitation and emission spectra are as follows image 3 shown.
example 3
[0024] Example 3 [Ca 0.8 Zn 0.2 TiO 3 ]: Pr 3+
[0025] The preparation method is the same as that of Example 1, except that butyl titanate is dissolved in 25 mL of ethylene glycol, and 0.75 mL of 0.0005 mol / L praseodymium nitrate solution is added. get [Ca 0.8 Zn 0.2 TiO 3 ]: Pr 3+ The complex phase titanate red phosphor, its excitation and emission spectra are as follows Figure 4 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com