A supramolecular hydrogel loaded with indomethacin and its preparation method
A technology of supramolecular hydrogel and indomethacin, which is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, and can solve problems that hinder the preparation of indomethacin injections. Achieve the effects of easy regulation, great application value, and increased load capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
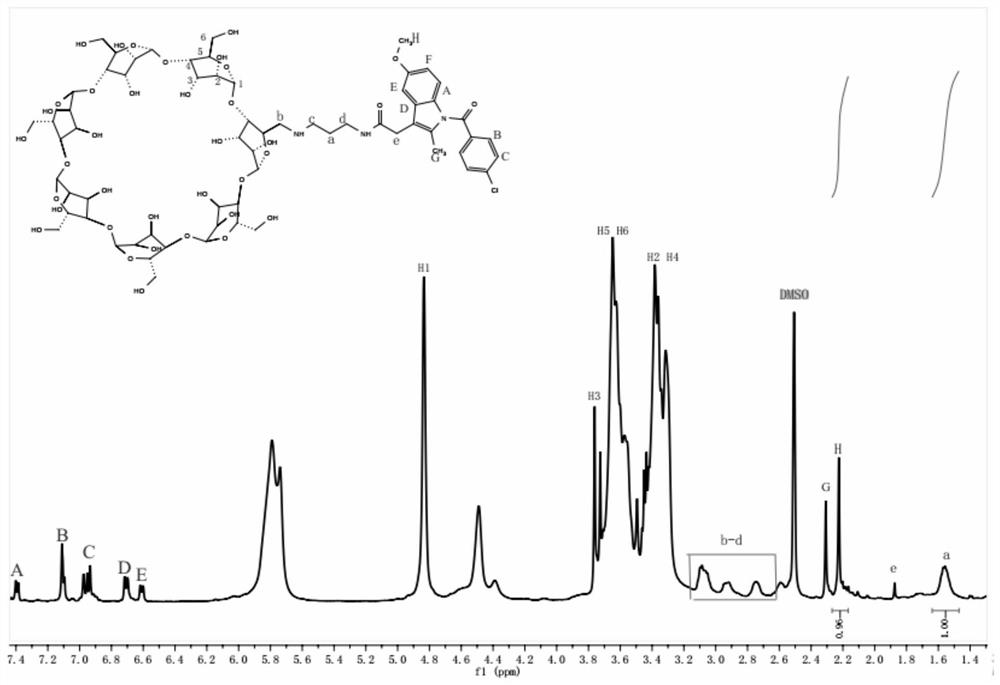
[0027]a. Accurately weigh β-cyclodextrin (210g, 0.19mol) and dissolve it in 1500mL of distilled water, stir it fully in an electric stirrer to make the solution into a milky white liquid, and control the temperature of the solution at about 15°C with a water bath , slowly added the prepared aqueous sodium hydroxide solution (34.4 g, 17.90 mol / 100 mL) into the liquid, and continued stirring for 1 h after observing that the cyclodextrin was completely dissolved. Accurately weigh p-toluenesulfonyl chloride (30.2g, 0.16mol) and quickly dissolve it in 90ml of acetonitrile, slowly drop the prepared p-toluenesulfonyl chloride acetonitrile solution into the cyclodextrin solution, pay attention to controlling the speed of solution dripping, constant temperature conditions Stirring was continued for 2.5 h. After static precipitation, the insoluble matter was removed by suction filtration under reduced pressure, and the pH value of the solution was slowly adjusted to 7.5 with 2M hydrochl...
Embodiment 2
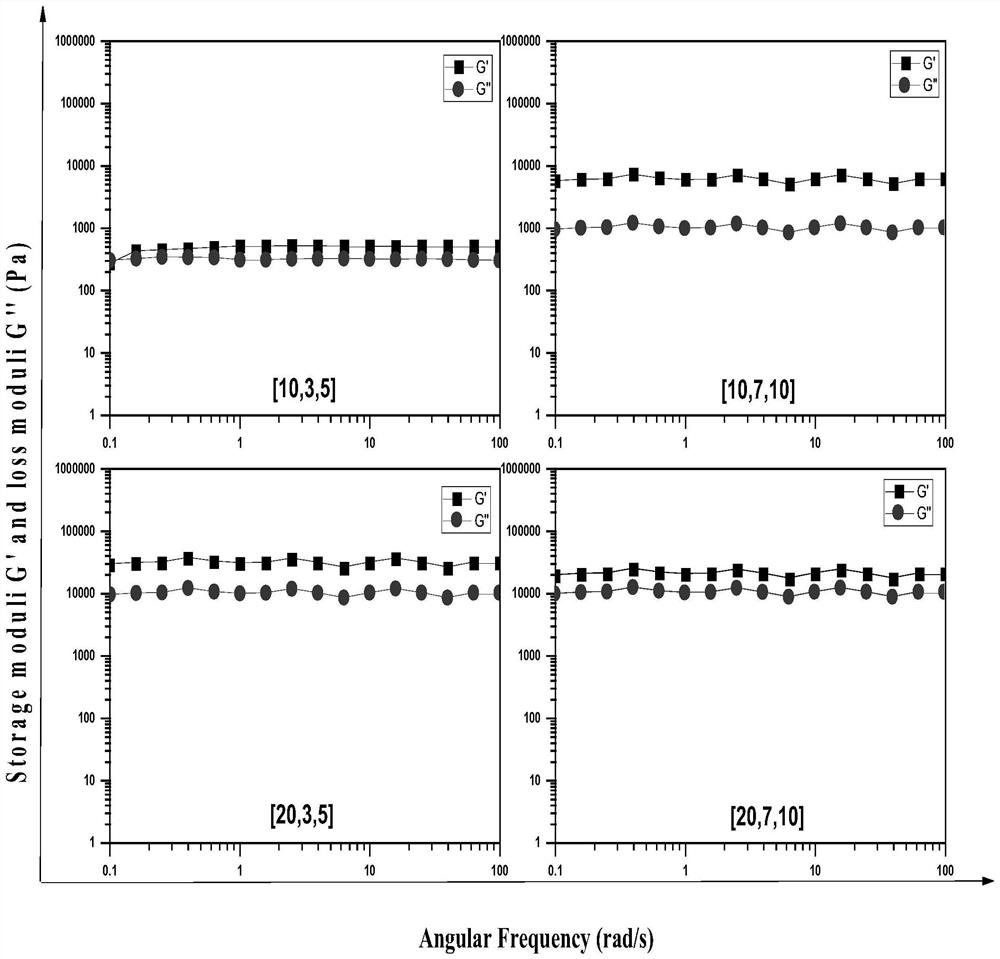
[0032] Take 0.35 g of the prodrug molecule indo-2N-β-CD prepared in Example 1 and 0.5 g of F127 in a reactor, add 5 ml of deionized water, and stir at 60° C. for 4 hours. Then 0.5g α-CD was added into the reaction system, stirring was continued at 60° C. for 4 h, and the whole reaction was carried out in the dark. After the reaction, the reaction vial was slowly cooled to room temperature, and after standing for 12 hours, it was freeze-dried for 12 hours to obtain a dry hydrogel drug-loading system. The mass / volume contents of F127, indo-2N-β-CD, and α-CD were 10%, 7%, and 10%, respectively.
Embodiment 3
[0034] Take 0.15 g of the prodrug molecule indo-2N-β-CD and 0.25 g of F127 prepared in Example 1 in a reactor, add 5 ml of deionized water, and stir at 60° C. for 4 h. Then, 1 g of α-CD was added into the reaction system, and the stirring was continued at 60° C. for 4 h, and the whole reaction was carried out in the dark. After the reaction, the reaction vial was slowly cooled to room temperature, and after standing for 12 hours, it was freeze-dried for 12 hours to obtain a dry hydrogel drug-loading system. The mass / volume contents of F127, indo-2N-β-CD, and α-CD were 20%, 3%, and 5%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com