*derivatives, materials containing the same and organic electroluminescent devices
A derivative, electromechanical technology, applied in the field of organic electroluminescent devices, can solve problems such as unstable thin films, accelerated device degradation, and poor device life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
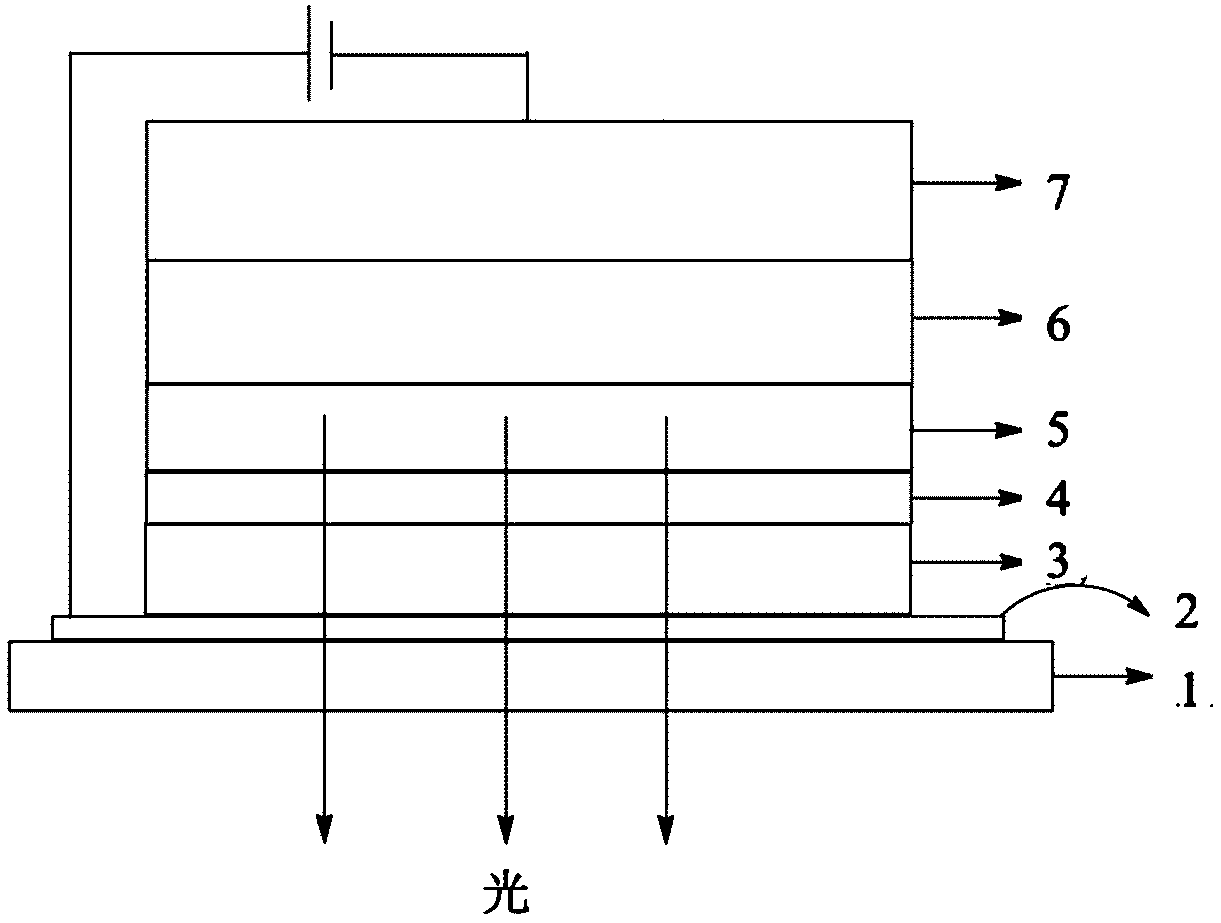
Image
Examples
Embodiment 1
[0088] Embodiment 1, the preparation of compound formula A-1
[0089] The first step, 11-(4-bromophenyl)-3-chloro The preparation, preparation route is as follows:
[0090]
[0091] The specific operation process of preparation:
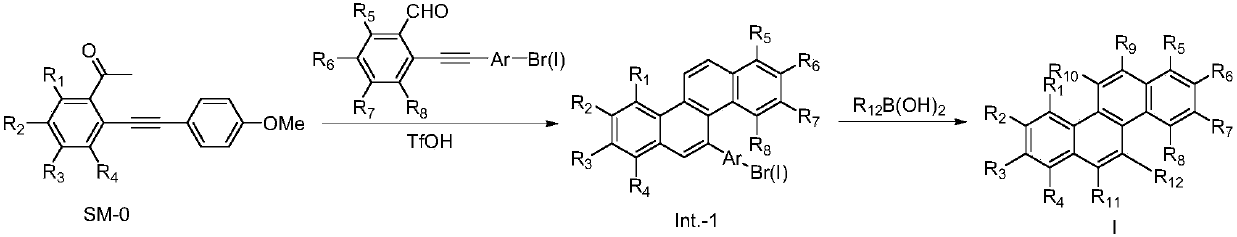
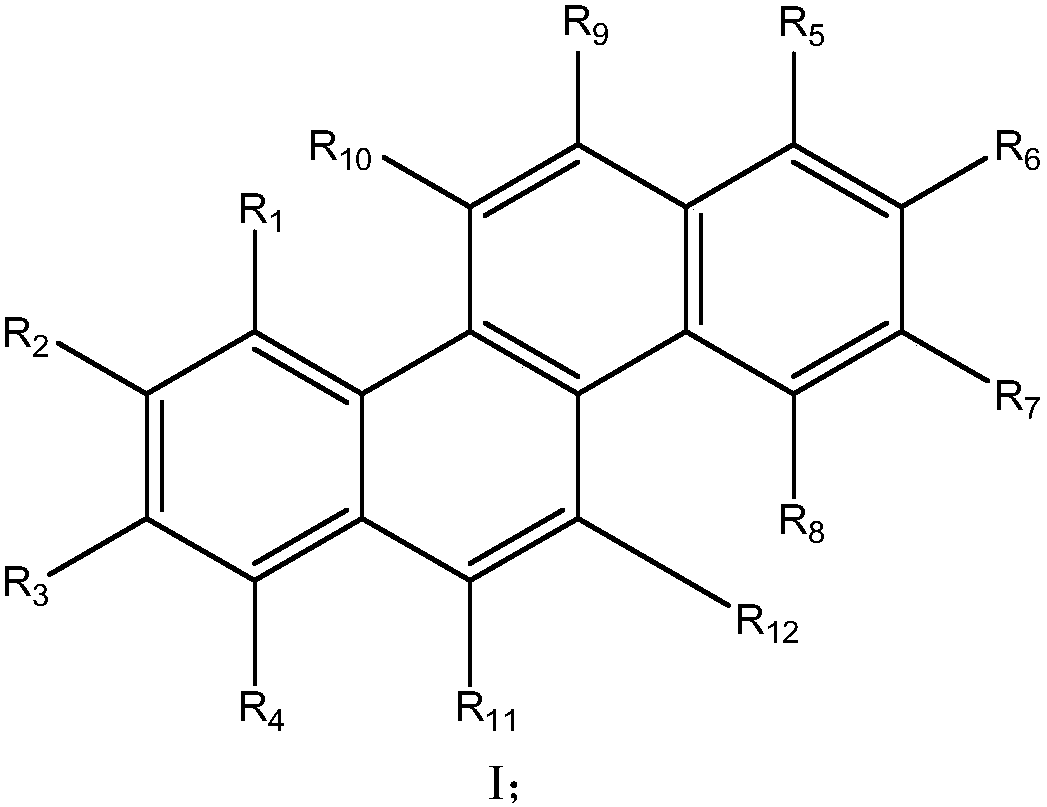
[0092] 20.0g (0.07mol) of raw material SM-0 was dissolved in 360ml of anhydrous dichloromethane, cooled to 0°C in an ice-salt bath, 13.5g (46.8mmol) of raw material SM-1 was added, and 11.2g (74.9mmol) of SM-1 was slowly added dropwise. ) of trifluoromethanesulfonic acid dissolved in dichloromethane, stirred for 1 hour, heated to 30 ° C, stirred for 18 hours, concentrated under reduced pressure to dryness, separated and purified with silica gel column, concentrated under reduced pressure to dryness, and obtained 13.2 g of white Solid, yield 45%.
[0093] The second step, the preparation of 2-bromo-4,4'-dibromomethylene biphenyl, the preparation route is as follows:
[0094]
[0095] The specific operation process of preparation:
[0096] ...
Embodiment 2
[0100] Embodiment 2, the preparation of compound formula A-17
[0101] The first step, intermediate 2,9-dichloro-5-(1-naphthyl) The preparation, preparation route is as follows:
[0102]
[0103] The specific operation process of preparation:
[0104] Referring to the preparation method in the first step of Example 1, SM-1 in the first step of Example 1 was replaced with SM-4 to obtain the intermediate Int.-2, a white solid, with a yield of 38%.
[0105] The second step, the preparation of compound formula A-17, the preparation route is as follows:
[0106]
[0107] The specific operation process of preparation:
[0108] The palladium catalyst Pd(PPh 3 ) 4 And the sodium carbonate of 10g (94.3mmol), then add the toluene of 60ml, the ethanol of 20ml and the water of 20ml, under the protection of nitrogen, heat up and reflux and stir reaction for 12 hours, cool to room temperature, extract with ethyl acetate, organic phase with Dry over sodium sulfate, filter, and c...
Embodiment 3
[0112] Embodiment 3, the preparation of compound formula A-33
[0113] The first step, intermediate 11-([1,1'-biphenyl]-2-yl)-2-chloro The preparation, preparation route is as follows:
[0114]
[0115] The specific operation process of preparation:
[0116] Referring to the preparation method of the first step of Example 1, SM-0 in the first step of Example 1 was replaced by SM-5, and SM-1 was replaced by SM-6 to obtain the intermediate Int.-3, a white solid, obtained as rate 47%.
[0117] The second step, the preparation of compound formula A-33, the preparation route is as follows:
[0118]
[0119] The specific operation process of preparation:
[0120] Referring to the preparation method of the second step of Example 2, replace Int.-2 in the second step of Example 2 with Int.-3, and replace phenylboronic acid with 2-phenylphenylboronic acid to obtain product A-33, a white solid , yield 88%.
[0121] Experimental data:
[0122] (1) 1 HNMR (δ, CDCl3): 9.06(d,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Film thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com