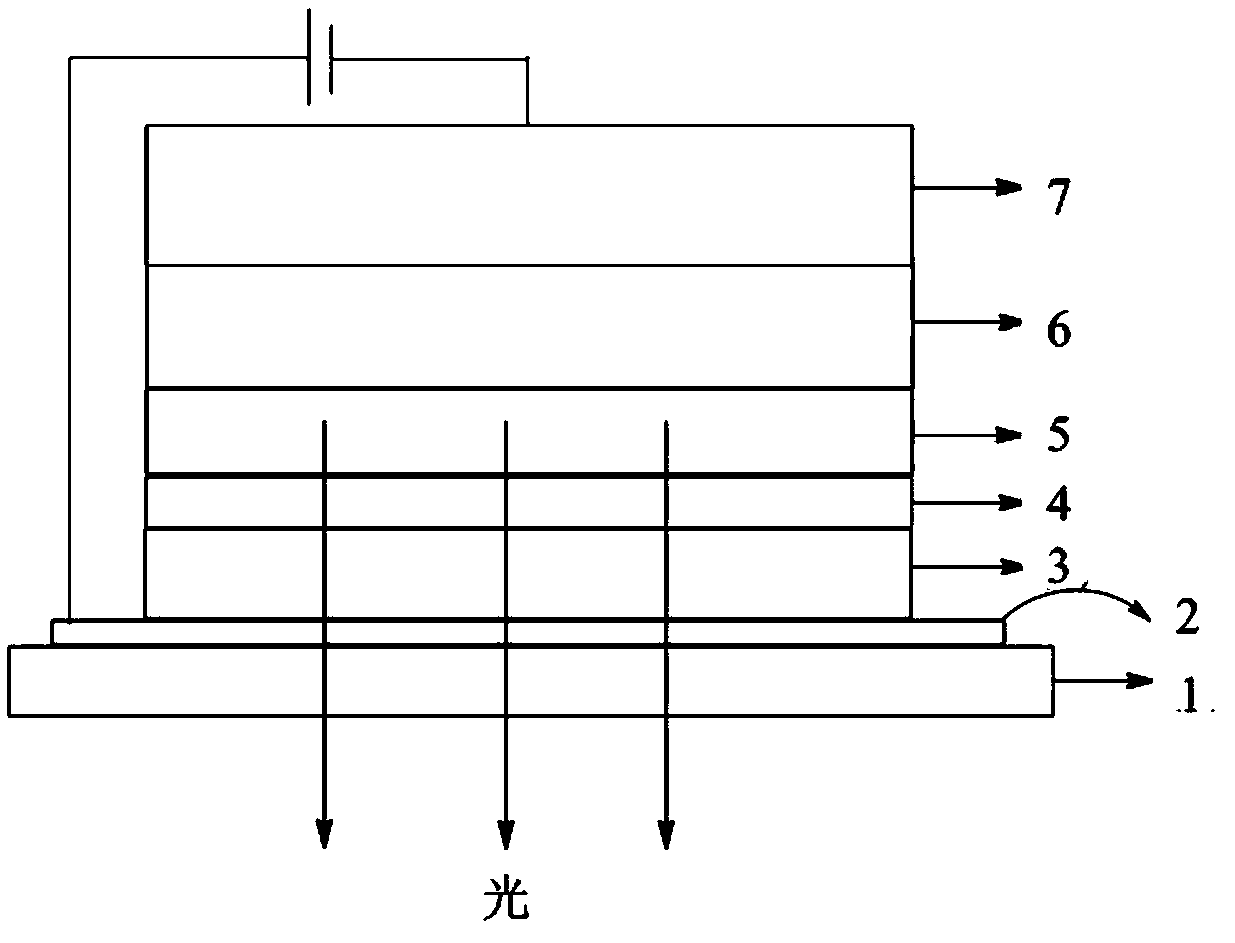
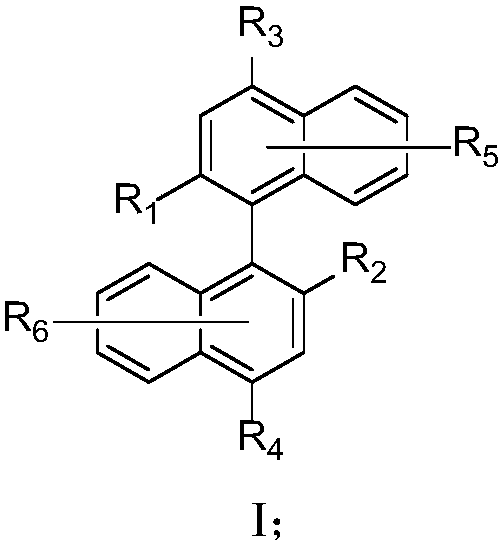
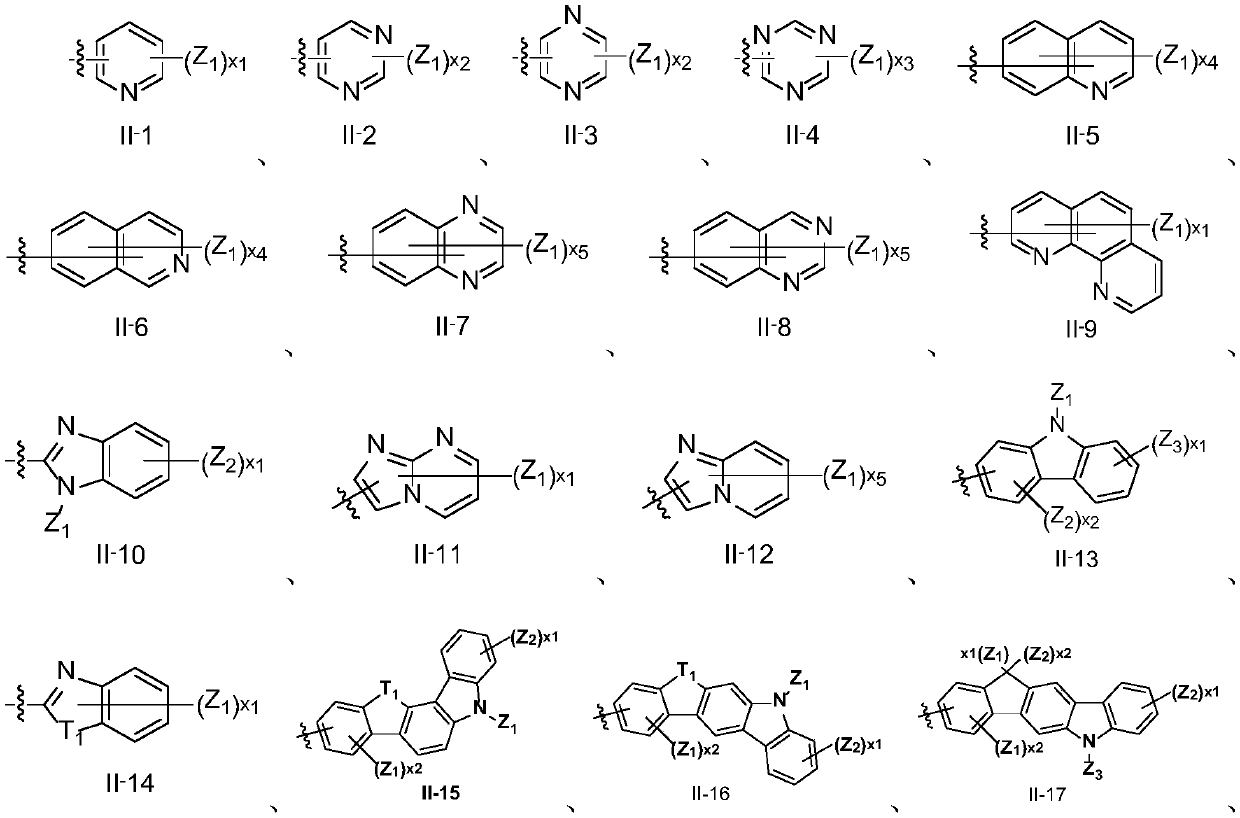
Binaphthyl derivative, and material and organic electroluminescent device which contain binaphthyl derivative
A technology of binaphthalene derivatives and organic light-emitting layer, applied in the field of binaphthalene derivatives, can solve the problems of accelerated device degradation, poor device life, unstable thin films, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Embodiment 1, the preparation of compound formula DN-01
[0091] The first step, the preparation of intermediate Int.-1, the preparation route is as follows:
[0092]
[0093] The specific operation process of preparation:
[0094]20.0g (69.93mol) of raw material SM-0 was dissolved in 300ml of anhydrous dichloromethane, 27.5g (0.35mol) of pyridine and 1.5g of DMAP were added, and 43.4g (0.15mol) of trifluoromethanesulfonium was slowly added dropwise Acid anhydride dissolved in dichloromethane solution, stirred at room temperature for 8 hours, added 300ml of water and stirred for 30 minutes, collected organic phase and concentrated to dryness under reduced pressure, separated and purified with silica gel column, concentrated to dryness under reduced pressure to obtain 36.5g of white solid, yield 95 %.
[0095] The second step, the preparation of intermediate Int.-2, the preparation route is as follows:
[0096]
[0097] The specific operation process of preparat...
Embodiment 2
[0110] Embodiment 2, compound formula DN-02, DN-04, DN-16, DN-49, DN-57, DN-60, DN-66, DN-80, DN-99, DN-100, DN-101, Preparation of DN-109
[0111] Referring to the synthesis method of Example 1, the compounds DN-02, DN-04, DN-16, DN-49, DN-57, DN-60, DN-66, DN-80, DN-99, DN-100, DN-101, DN-109, that is, the method steps are the same as in Example 1, the only difference is that according to the desired product, different reactants are used to replace the phenylboronic acid in the second step of Example 1 according to actual needs, replacing Example 1 The phenylboronic acid of the 4th step; And change the quality consumption of this compound according to molar weight.
Embodiment 3
[0112] Embodiment 3, the preparation of compound formula DN-36
[0113] The first step, the preparation of intermediate Int.-4, the preparation route is as follows:
[0114]
[0115] The specific operation process of preparation:
[0116] Dissolve 10.0g (24.6mmol) of the intermediate Int.-2 in the second step of Example 1 with 200ml of dichloromethane, cool down to 0°C in an ice-salt bath, and slowly add 3.9g (24.6mmol) of bromine to dissolve in 10ml The solution of dichloromethane was stirred and reacted for 6 hours, 20ml of saturated aqueous sodium bisulfite solution was added, stirred and reacted for 10 minutes, the organic phase was collected, concentrated under reduced pressure to dryness, separated and purified by silica gel column, concentrated under reduced pressure to dryness, and 10.2g White solid, yield 86%.
[0117] The second step, the preparation of compound formula DN-36, the preparation route is as follows:
[0118]
[0119] The specific operation proc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com