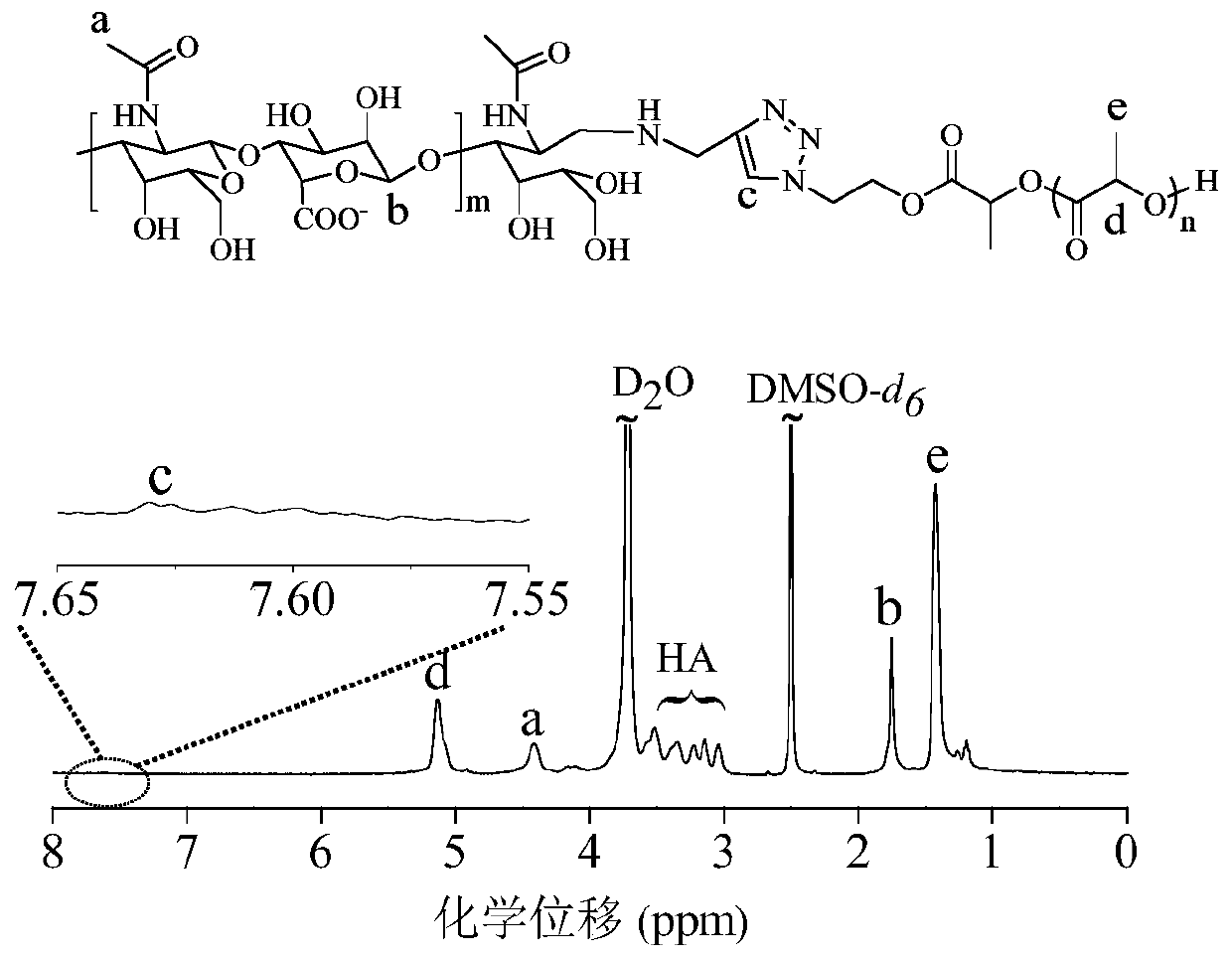
Star-shaped polymer containing lipoyl group at the end, preparation method thereof, polymer nanoparticle prepared therefrom and application thereof
A star polymer, lipoyl technology, applied in biocompatible polymer materials, polymer nanoparticles and applications in targeted nanomedicine, star biocompatible polymer field, can solve the lack of Low toxic and side effects and high-efficiency nano-medicines, etc., to achieve excellent biodegradability, increase enrichment, and high loading efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
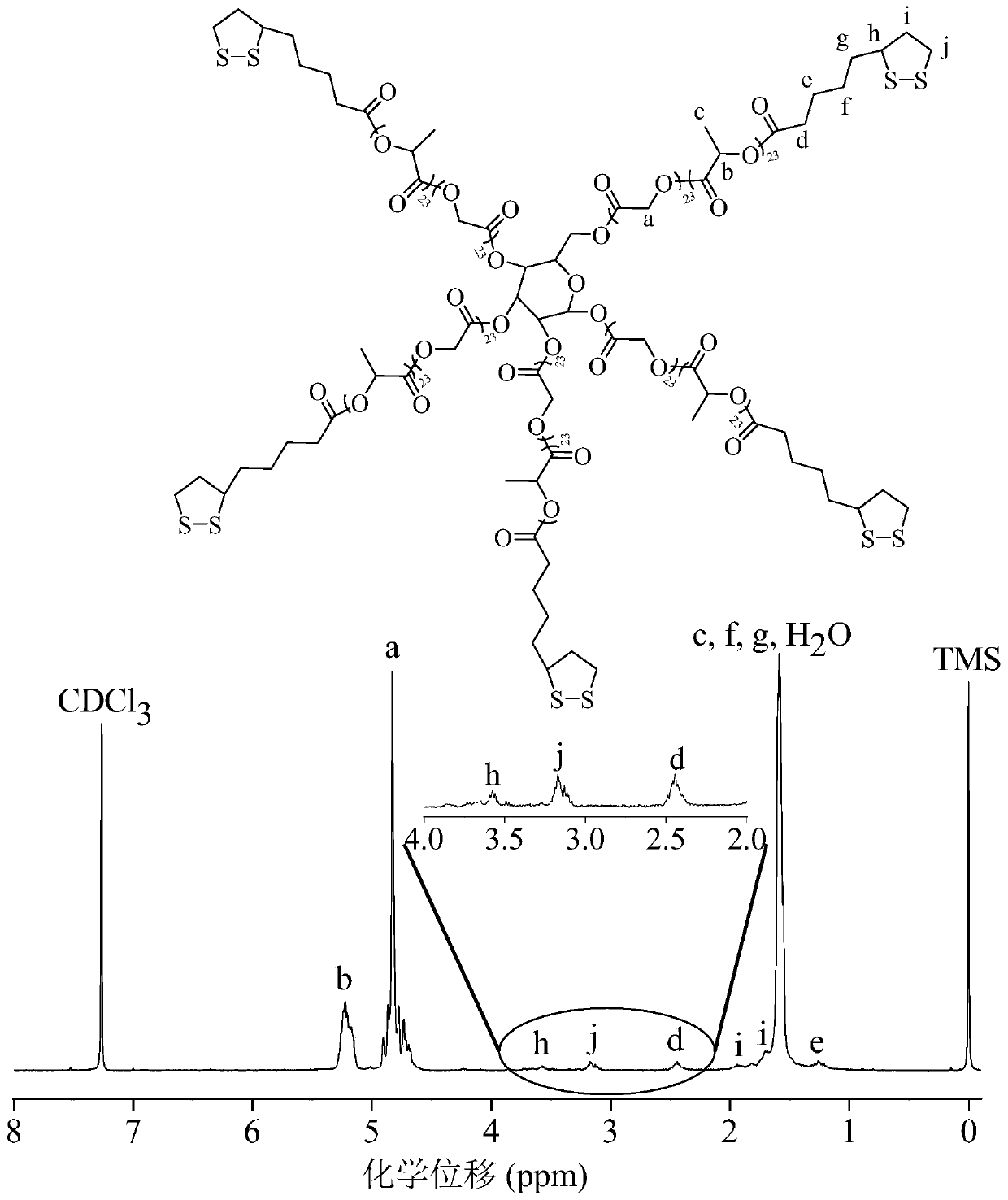
[0055] Example 1 Synthesis of star polymer with lipoyl group in side chain
[0056] Synthetic star polymer and linear polymer
[0057] The star polymer can be synthesized by using polyhydroxy glucose as an initiator and under the catalysis of stannous octoate to initiate the ring-opening polymerization reaction of lactide and glycolide. In N 2 Under the environment, add 0.18 g (1 mmol) polyhydroxy glucose (manufacturer: Sigma-Aldrich), 7.5 g (52 mmol) lactide and 7.5 g (65 mmol) glycolide into a closed reaction flask, and then add 4.73 Add mg of catalyst stannous octoate to the reaction flask and mix all materials evenly. Then vacuum the reaction flask-replace N 2 Three times, finally the reaction flask was evacuated for 30 minutes, and the reaction flask was sealed. The polymerization reaction was carried out in a vacuum box at 160°C for 8 hours. The crude product was dissolved in dichloromethane, then precipitated in ice methanol, filtered with suction and dried in vacuum to ...
Embodiment 2
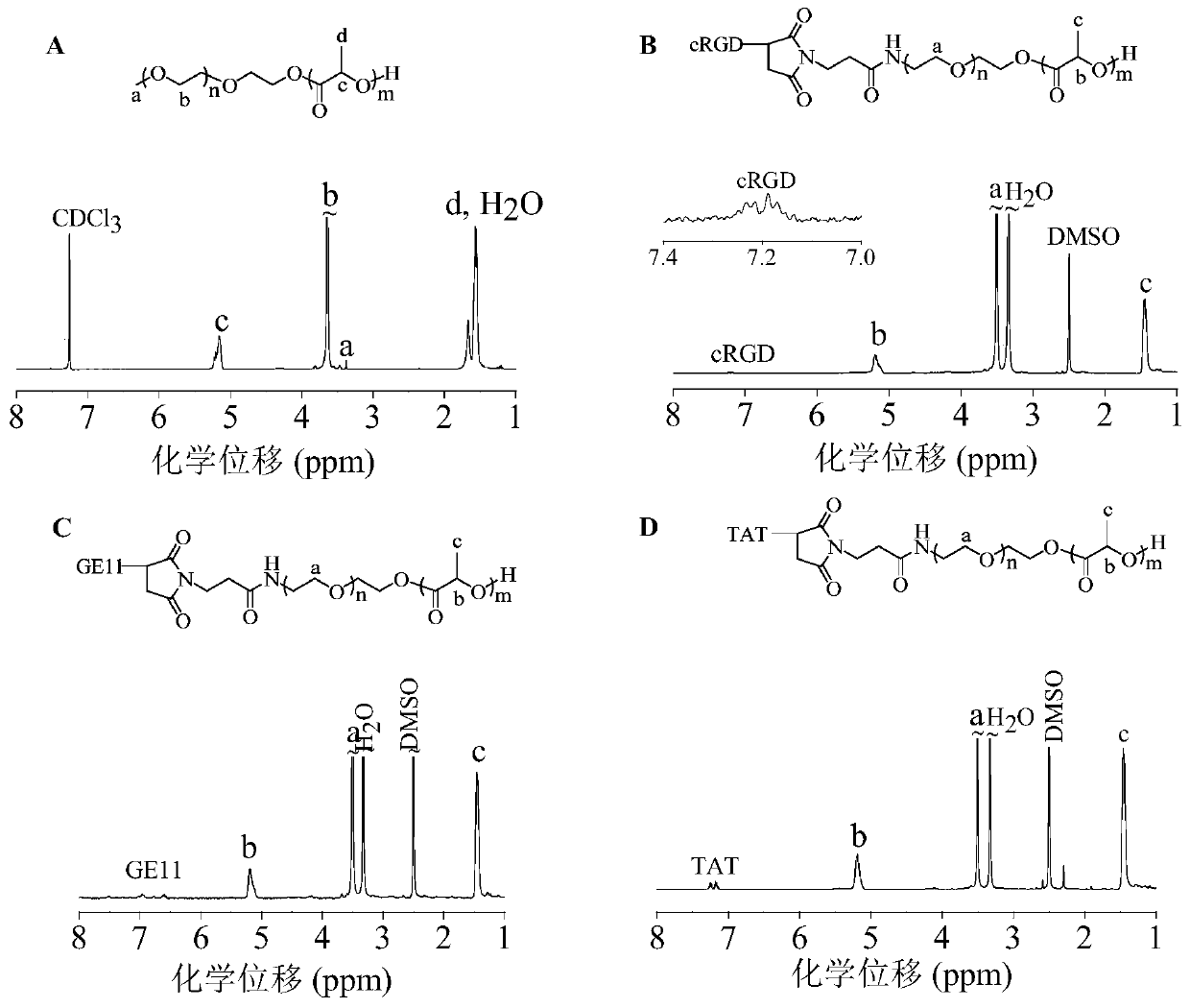
[0061] Example 2 Synthesis of amphiphilic polymer PEG-PDLLA
[0062] The amphiphilic polymer PEG-PDLLA can be prepared by the ring-opening polymerization of D,L-lactide initiated by the macromolecular initiator PEG. In N 2 Under the environment, add 2.5 mL PEG ( M n =5.0 kg / mol, 0.5 g, 0.1 mmol) and D,L-lactide (0.4g, 2.8 mmol) in anhydrous toluene solution, quickly add 0.5 mL (0.2 mol / L) stannous octoate in toluene stock liquid. After reacting in a constant temperature oil bath at 110°C for 48 h, the reaction was terminated by adding glacial acetic acid. Subsequently, the product was precipitated in ice ether, filtered with suction and dried in vacuum to obtain PEG-PDLLA with a yield of 88.9%. 1 H NMR (600 MHz, CDCl 3 ): δ 5.16 (-C H (CH 3 )O- ), 3.65 (-C H 2 C H 2 O-), 3.38 (C H 3 O-), 1.56 (-CH(C H 3 )O-), see figure 2 (A). M n ( 1 HNMR) = 8.9 kg / mol, M n (GPC) = 15.9 kg / mol, M w / M n (GPC) = 1.3.
Embodiment 3
[0063] Example 3 Synthesis of amphiphilic targeting polymer cRGD-PEG-PDLLA
[0064] The targeting polymer cRGD-PEG-PDLLA was obtained through a two-step reaction. First, synthesize maleimide functionalized amphiphilic polymer MAL-PEG-PDLLA, and then further synthesize cRGD polypeptide modified amphiphilic polymer cRGD-PEG- by Michael addition of MAL and sulfhydryl peptide cRGD-SH. PDLLA. Maleimide functionalized MAL-PEG-PDLLA is prepared by MAL-PEG-initiated ring-opening polymerization of D,L-lactide. In N 2 Under the environment, add 2.5 mL MAL-PEG ( M n =5.0 kg / mol, 0.5 g, 0.1 mmol) and D,L-lactide (0.4 g, 2.8 mmol) in anhydrous toluene solution, quickly add 0.5mL (0.2 mol / L) stannous octoate in toluene stock liquid. After reacting in a constant temperature oil bath at 110°C for 48 h, glacial acetic acid was added to terminate the reaction. The product was then precipitated in ice ether, filtered with suction and dried in vacuum to obtain MAL-PEG-PDLLA. Then dissolve MAL-P...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com