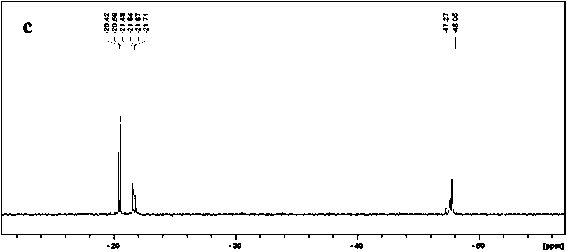
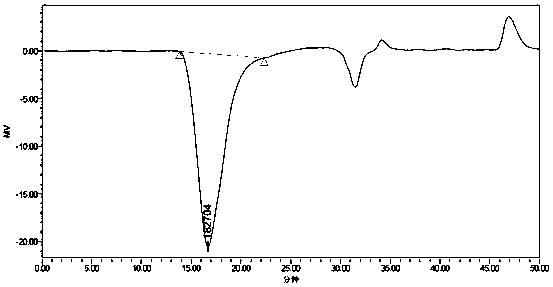
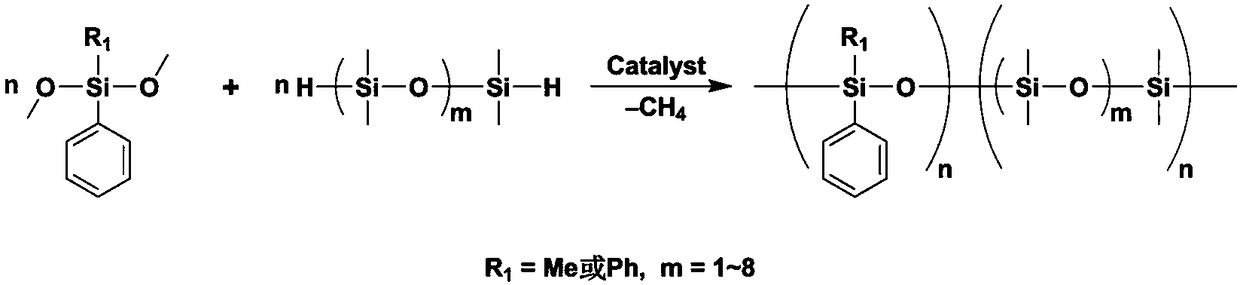
Preparation method of methylphenyl silicone oil with phenyl groups of medium and high content
A technology of methyl phenyl silicone oil and high phenyl group is applied in the field of preparation of medium and high phenyl content methyl phenyl silicone oil, which can solve the problems of deviation of phenyl group content, small steric hindrance, weak production capacity and scale, etc. Low content of cyclic products, mild reaction conditions, and the effect of ensuring excellent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Preparation of 1,1,3,5,5-pentamethyl-3-phenyltrisiloxane
[0039] 191.2g (1mol) MePhSiCl 2 with 283.9g (3mol) Me 2 After the HSiCl was mixed evenly, it was added dropwise into a two-phase system consisting of 1000 mL of water and 200 mL of toluene at 0 °C and fully stirred. After the dropwise addition, the reaction mixture was left standing, and the organic phase was separated after complete separation, and then the organic phase was washed to neutrality, and then the fraction at 88-89°C / 800Pa was collected by vacuum distillation to obtain 222.7g of 1,1 , 3,5,5-pentamethyl-3-phenyltrisiloxane, yield 82.3%.
[0040] (2) Preparation of silicone oil
[0041] 91.2g (0.5mol) MePhSi(OMe) 2 , 0.193g (0.25mmol) catalyst three (perfluoronaphthyl) borane B (C 10 f 7 ) 3 After mixing with 91g of toluene, at 25°C and fully stirred, add 67.7 g (0.25mol) of the product 1,1,3,5,5-pentamethyl-3-phenyltrisilane dropwise in step (1) The mixture of oxane and 17g toluene, prope...
Embodiment 2
[0043] (1) Preparation of 1,1,5,5-tetramethyl-3,3-diphenyltrisiloxane
[0044] 253.2g (1mol) Ph 2 SiCl 2 with 378.5g (4mol) Me 2 After the HSiCl was mixed evenly, it was added dropwise to a two-phase system consisting of 2300 mL of water and 300 mL of chloroform at 25 °C and fully stirred. After the dropwise addition, the reaction mixture was left to stand, and the organic phase was separated after the layers were completely separated, and then the organic phase was washed to neutrality, and then the fraction at 128-129°C / 300Pa was collected by vacuum distillation to obtain 279.4g 1,1, 5,5-tetramethyl-3,3-diphenyltrisiloxane, yield 84.0%.
[0045] (2) Preparation of silicone oil
[0046] 122.2g (0.5mol) Ph 2 Si(OMe) 2 , 0.256g (0.5mmol) catalyst three (pentafluorophenyl) borane B (C 6 f 5 ) 3 After mixing with 122g of chloroform, at 30°C and fully stirred, 83.2g (0.25mol) of the product 1,1,5,5-tetramethyl-3,3-diphenyltri For siloxane, properly adjust the dropping ra...
Embodiment 3
[0048] (1) Preparation of 1,1,3,5,5-pentamethyl-3-phenyltrisiloxane
[0049] 191.2g (1mol) MePhSiCl 2 with 189.3g (2mol) Me 2 After the HSiCl was mixed evenly, it was added dropwise to a two-phase system consisting of 2100 mL of water and 210 mL of diethyl ether at -20 °C under sufficient stirring conditions. After the dropwise addition, the reaction mixture was left to stand, and the organic phase was separated after it was completely separated, and then the organic phase was washed to neutrality, and then the fraction at 88-89°C / 800Pa was collected by vacuum distillation to obtain 212.2g of 1,1 , 3,5,5-pentamethyl-3-phenyltrisiloxane, yield 78.4%.
[0050] (2) Preparation of silicone oil
[0051] 105.2g (0.5mol) MePhSi (OEt) 2 , 0.512g (1mmol) Catalyst B (C 6 f 5 ) 3 After mixing with 27g of dichloromethane, 101.5g (0.375mol) of the product 1,1,3,5,5-pentamethyl-3-phenyl was added dropwise at 0°C under sufficient stirring conditions The mixture of trisiloxane and 102...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com