HPTS series derivatives and synthesis method
A synthesis method and derivative technology, applied in the field of HPTS series derivatives and synthesis, can solve the problems of strong water solubility of HPTS, easy leakage of fluorescent dyes, narrow application range, etc., and achieve increased hydrophobicity, broadened application range, and low toxicity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
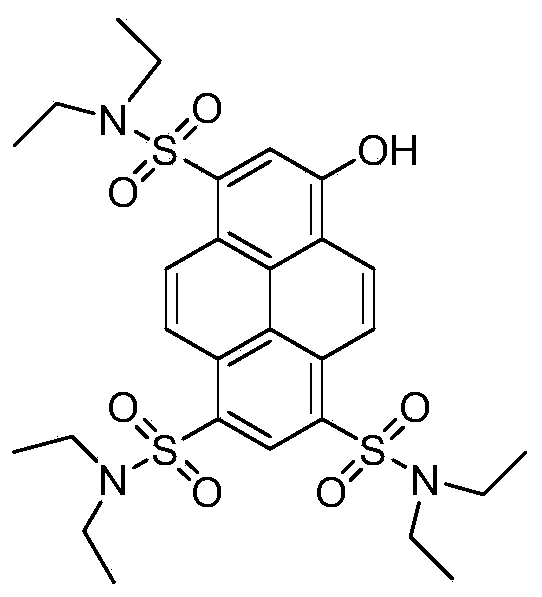
[0044] In this example, 8-hydroxypyrene-1,3,6-trisodium trisulfonate (HPTS) is used as a precursor to synthesize 8-hydroxy-1,3,6-trisulfdiethylamine (HPTS-diethylamine), and the synthesis steps are as follows :
[0045] 1) Put HPTS and phosphorus oxychloride into reaction according to 1:3 equivalent, DMF catalyzes, heats and refluxes for 12 hours;
[0046] 2) Slowly introduce the reaction product in step 1) into ice water and stir to precipitate a solid, and obtain HPTS-SO by suction filtration 2 Cl. Yield 90%.
[0047] 3) the HPTS-SO obtained in step 2) 2 Cl was dissolved in an appropriate amount of tetrahydrofuran (THF) to prepare a concentration of 0.3mmol mL -1 solution A, dimethylamine was dissolved in an appropriate amount of tetrahydrofuran (THF) according to 3 times the equivalent, and prepared to a concentration of 0.5mmol mL -1 solution B.
[0048] 4) Slowly add solution B dropwise to solution A and react at room temperature for 24 hours, then spin evaporate to...
Embodiment 2
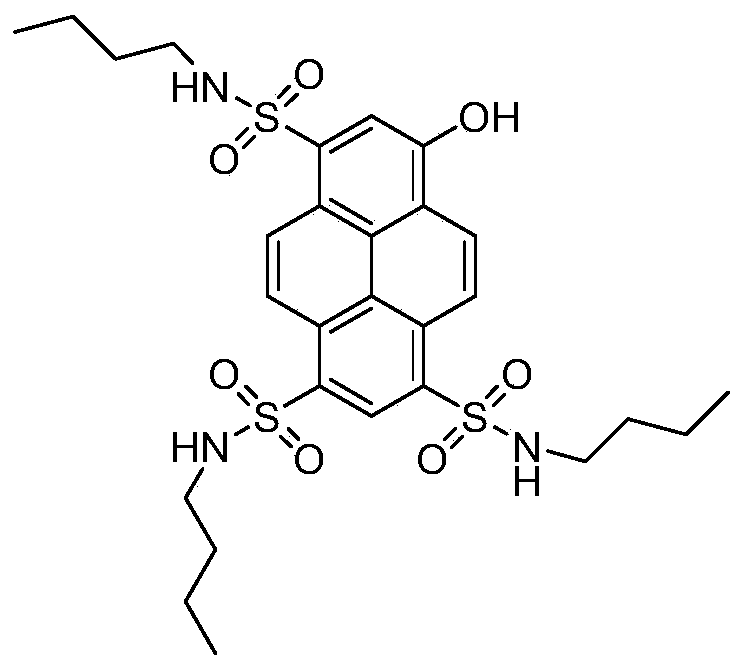
[0053] In this embodiment, 8-hydroxypyrene-1,3,6-trisodium trisulfonate (HPTS) is used as a precursor to synthesize HPTS-n-butylamine; the steps of its synthesis method are as follows:
[0054] 1) Put HPTS and phosphorus oxychloride into reaction according to the equivalent ratio of 1:3.1, catalyze with DMF, and heat to reflux for 12 hours.
[0055] 2) Slowly introduce the reaction product in step 1) into ice water and stir to precipitate a solid, and obtain HPTS-SO by suction filtration 2 Cl. Yield 90%.
[0056] 3) the HPTS-SO obtained in step 2) 2 Cl was dissolved in an appropriate amount of tetrahydrofuran (THF) to prepare a concentration of 0.3mmol mL -1 The solution A of the solution A was prepared by dissolving 3.1 equivalents of n-butylamine in an appropriate amount of tetrahydrofuran (THF) to a concentration of 0.5 mmol mL -1 solution B.
[0057] 4) Slowly add solution B to solution A drop by drop, react at room temperature for 24 hours, and spin evaporate to obta...
Embodiment 3
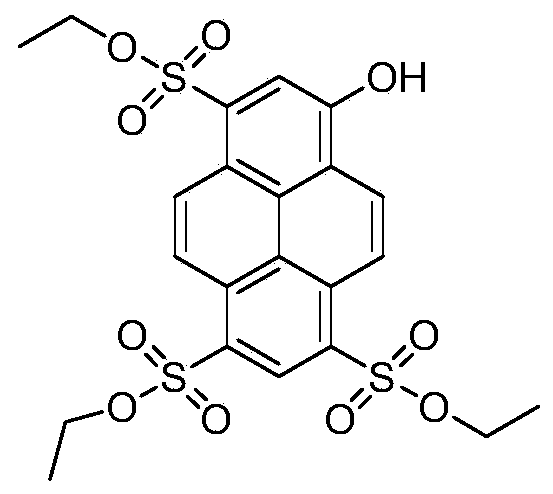
[0062] In this embodiment, 8-hydroxypyrene-1,3,6-trisodium trisulfonate (HPTS) is used as a precursor to synthesize HPTS-ethyl ester; the steps of its synthesis method are as follows:
[0063] 1) Put HPTS and phosphorus oxychloride into the reaction according to the equivalent ratio of 1:4, DMF catalyzes, heat and reflux for 12 hours.
[0064] 2) Slowly introduce the reaction product in step 1) into ice water and stir to precipitate a solid, and obtain HPTS-SO by suction filtration 2 Cl. Yield 90%.
[0065] 3) the HPTS-SO obtained in step 2) 2 Cl was dissolved in tetrahydrofuran (THF) to prepare a concentration of 0.3mmol mL -1 solution A, according to 4 times the equivalent of absolute ethanol dissolved in tetrahydrofuran (THF), prepared to a concentration of 0.5mmol mL -1 solution B.
[0066] 4) Slowly add solution B to solution A drop by drop, react at room temperature for 24 hours, and rotate to obtain the product. After column separation, the pure product of 8-hydrox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com