Preparation method of salbutamol intermediate V hydrochloride
A technology of albuterol and intermediates, applied in the field of preparation of albuterol intermediate V hydrochloride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
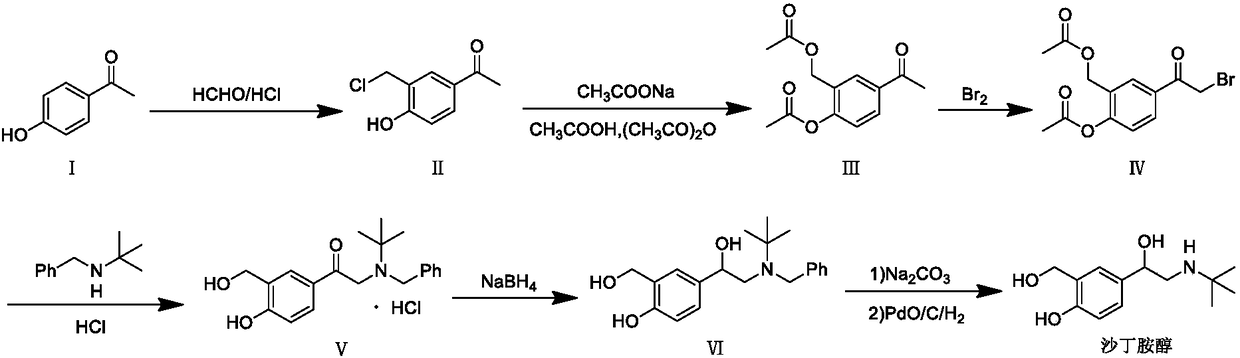
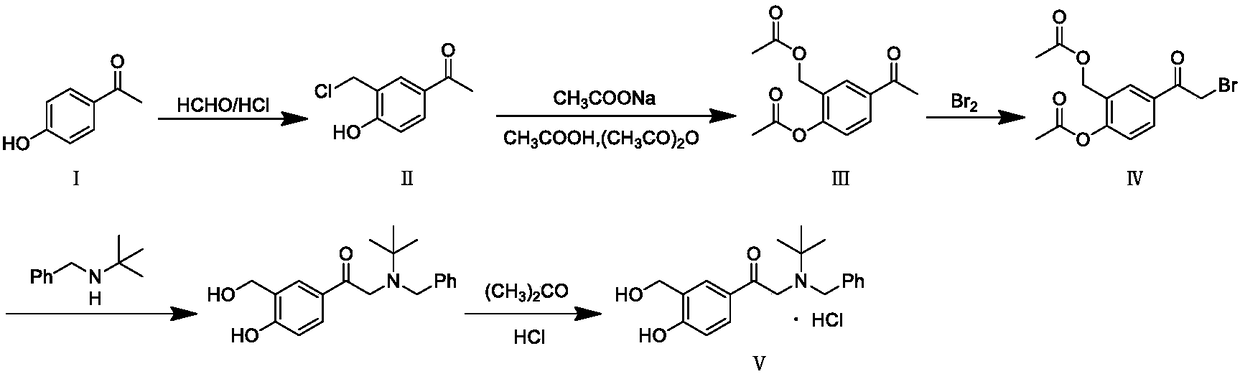
[0021] A preparation method of salbutamol intermediate V hydrochloride is as follows: intermediate IV and N-benzyl tert-butylamine are refluxed in the reaction solvent ethyl acetate, filtered, acidified, and the product is extracted into the water layer, hydrolyzed under reflux, Alkalinization, extraction with ethyl acetate, concentration to obtain the free base of intermediate V, the concentrated free base was dissolved in an organic solvent, and aqueous hydrochloric acid was added dropwise to obtain the hydrochloride of intermediate V. The process route is as follows:
[0022]
Embodiment 1
[0023] Embodiment one: the preparation of intermediate IV
[0024] Referring to the patent US3642896, add intermediate III to chloroform, stir and cool to 20°C, and then dissolve bromine in the solution. After completion, water was added to the mixture, and the chloroform layer was separated, washed with water and dried over sodium sulfate. Chloroform was evaporated in vacuo to afford Intermediate IV.
Embodiment 2
[0025] Embodiment two: the preparation of intermediate V hydrochloride
[0026] Dissolve 20g of intermediate IV in 300ml of ethyl acetate, add 25.9g of N-benzyl tert-butylamine, heat to reflux for 15 hours, cool down to 20-25°C, filter, add 32g of hydrochloric acid aqueous solution diluted with 300ml of water to the filtrate, stir and extract, separate solution to obtain the water layer, heat the water layer to reflux for 4 hours, lower the temperature to 20-25°C, add 300ml of ethyl acetate, adjust the pH to 9 with sodium carbonate, separate the layers to obtain the ethyl acetate layer, and concentrate the ethyl acetate under reduced pressure at 50°C After the solution was added, 150ml of tetrahydrofuran was added, 2.4g of hydrochloric acid was added dropwise, kept at 20-30°C and stirred for 1h, cooled to 0-5°C and stirred for 1h, filtered, and dried under reduced pressure at 50°C to obtain 14.62g of intermediate V hydrochloride with a purity of 97.5 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com