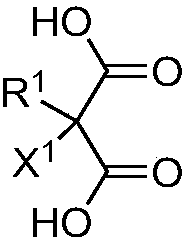
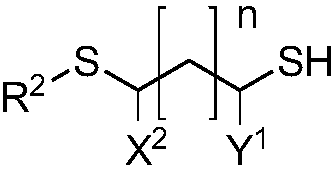
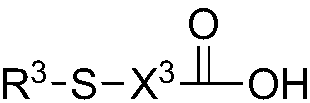Quantum dot having organic ligand and application thereof
A technology of quantum dots and ligands, which is applied in the field of quantum dots with organic ligands and their uses, can solve problems such as oxygen oxidation, and achieve the effect of excellent oxidation stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0208] Synthesis example 1: Synthesis of quantum dots with InP core alone
[0209] In a three-necked flask (3-neck flask), 0.05839 g of indium acetate, 0.12019 g of oleic acid, and 10 mL of 1-octadecene (ODE) were charged. After the flask was subjected to a degassing process at 110° C., 100 mTorr (mTorr) for 30 minutes while stirring, it was heated at a temperature of 270° C. under an inert gas until the solution was clear.
[0210] As a phosphorus (P) precursor, prepare 0.025054 g of tris(trimethylsilyl)phosphine, add 0.5 mL of 1-octadecene and 0.5 mL of tri-n-octylphosphine, stir, and quickly Pour into the flask which is heated at 270 °C. After reacting for 1 hour, it was rapidly cooled to terminate the reaction. Next, when the temperature of the flask reached 100° C., 10 mL of toluene was injected, and then transferred to a 50 mL centrifuge tube. After adding 10 mL of ethanol, it was purified twice by precipitation and redispersion methods. The purified InP core nanopar...
Synthetic example 2
[0211] Synthesis Example 2: Synthesis of InP / ZnS Core-Shell Quantum Dots
[0212] 3.669 g of zinc acetate, 20 mL of oleic acid, and 20 mL of 1-octadecene were placed in a three-necked flask, and after a 30-minute degassing process at 110° C. and 100 mTorr while stirring, the After heating at a temperature of 270 °C until the solution was clear, it was cooled to 60 °C to obtain a transparent precursor solution in the form of zinc oleate.
[0213] Add 0.6412g of sulfur and 10mL of tri-n-octylphosphine into a three-necked flask, heat at 80°C while stirring in an inert gas atmosphere until the solution is clear, then cool it to room temperature to obtain TOP:S form S precursor solution.
[0214] Put the pre-prepared InP core nanoparticle solution in Synthesis Example 1 into another three-necked flask, adjust the temperature of the flask to 300° C., and quickly inject 0.6 ml of the pre-prepared zinc precursor solution through a syringe. Then, 0.3 mL of the pre-prepared S precurso...
Embodiment 1-1
[0215] Example 1-1: Manufacturing of InP core-only quantum dots with ligands shown in Chemical Formula 5
[0216] Disperse 1 g of InP core single quantum dots in Synthesis Example 1 with oleic acid bound to the surface in 10 mL of toluene solution, add 0.5 g of the new ligand of malonic acid derivative shown in Chemical Formula 5, and stir for more than 30 minutes. In this process, the new ligand shown in Chemical Formula 5 replaces the oleic acid on the surface of the core alone quantum dot. Next, add 10ml of ethanol to the mixed solution of the quantum dot-new ligand conjugate and the unreacted ligand to make the quantum dot-new ligand conjugate condense. The condensed quantum dot-neoligand conjugates were separated from the oleic acid and unreacted ligands shed from the quantum dots by centrifugation (8000rpm, 30min). Next, the quantum dot-neoligand conjugate was dispersed in toluene at a concentration of 0.1 g / ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com