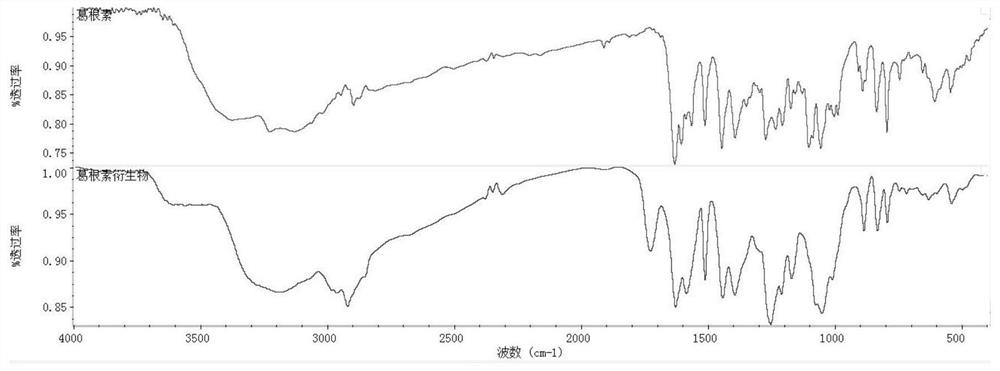
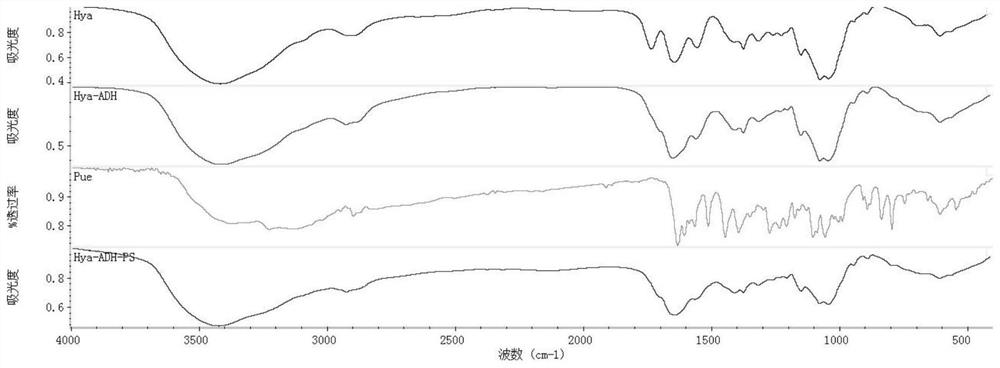
A kind of preparation method of puerarin hyaluronic acid nano-micelle
A technology of hyaluronic acid and nano-micelle, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc. It can solve the problems of poor fat solubility and water solubility, and achieve high drug loading , The preparation technology is simple and stable, and the effect of convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] First add hyaluronic acid into deionized water to fully dissolve, and then make a solution with a mass concentration of 0.4%, and add catalysts DCC and HOBt to it to prepare solution I. Then adipic acid dihydrazide (ADH) was added into deionized water to dissolve to obtain solution II with a mass concentration of 1%. Add solution I dropwise to solution II under stirring condition, by adding pH adjusting reagent-hydrochloric acid to adjust the pH of the system to pH4, controlling the reaction temperature at 5°C, stirring speed at 1000rpm and reaction time of 24 hours, the hyaluronic acid-ADH intermediate is obtained and the mixed solution of the catalyst, and then separate and dry it to obtain the hyaluronic acid-ADH intermediate of the present invention; under stirring conditions, an appropriate amount of hyaluronic acid-ADH intermediate and puerarin are fully dissolved, and then the catalyst EDC is added thereto And solutions such as DMAP, by adding pH adjustment reage...
Embodiment 2
[0035]Partially deacetylated hyaluronic acid was added into deionized water to fully dissolve it, and then a solution with a mass concentration of 1% was prepared, and catalysts EDC and NHS were added to it to obtain solution I. Then adipic acid dihydrazide (ADH) was added into deionized water to dissolve to obtain solution II with a mass concentration of 8%. Add solution I dropwise to solution II under stirring conditions, adjust the pH of the system to pH 10 by adding a pH adjusting reagent-ammonium bicarbonate, control the reaction temperature at 25°C, the stirring speed at 5000rpm and the reaction time for 12 hours to obtain partially deacetylated transparent The mixed solution of hyaluronic acid-ADH intermediate and catalyst is then separated and dried to obtain the hyaluronic acid-ADH intermediate of the present invention; under stirring conditions, an appropriate amount of deacetylated hyaluronic acid-ADH intermediate and amber Ester puerarin is fully dissolved, and the...
Embodiment 3
[0037] First add methyl hyaluronate into deionized water to fully dissolve, then prepare a solution with a mass concentration of 1.2%, and add catalysts PyBop and DIEA to it to prepare solution I. Then adipic acid dihydrazide (ADH) was added into deionized water to dissolve to obtain a solution II with a mass concentration of 10%. Add solution I dropwise to solution II under stirring conditions, adjust the pH of the system to pH 12 by adding a pH adjustment reagent-sodium hydroxide, control the reaction temperature at 60°C, the stirring speed at 10,000 rpm and the reaction time for 1 hour to obtain methyl hyaluronate - the mixed solution of ADH intermediate and catalyst, then it is separated and dried to obtain methyl hyaluronate-ADH intermediate of the present invention; under stirring conditions, an appropriate amount of methyl hyaluronate-ADH intermediate and succinic acid Fully dissolve the ester puerarin, then add catalysts such as DIC and DMAP to it, adjust the pH of the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com