Bleaching working fluid and application thereof
A technology of working liquid and ligand, which is applied in the field of bleaching, and can solve the problems of poor wettability of fabrics, high temperature, and strong alkali treatment time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0107] In the present invention, the preparation method of the metal complex preferably comprises the following steps:
[0108] Mix iminodiacetic acid derivatives, nitrogen-containing heterocyclic ligands, soluble metal salts and solvents, and undergo a coordination reaction in a protective atmosphere to obtain metal complexes.
[0109] In the present invention, the solvent is preferably one or more of water, methanol, ethanol, acetonitrile, acetone, N,N-dimethylformamide, tetrahydrofuran, chloroform, methylene chloride, benzene and ethyl acetate ; When the solvent is two or more of the above specific selections, the present invention does not have any special limitation on the mass ratio of the specific substances.
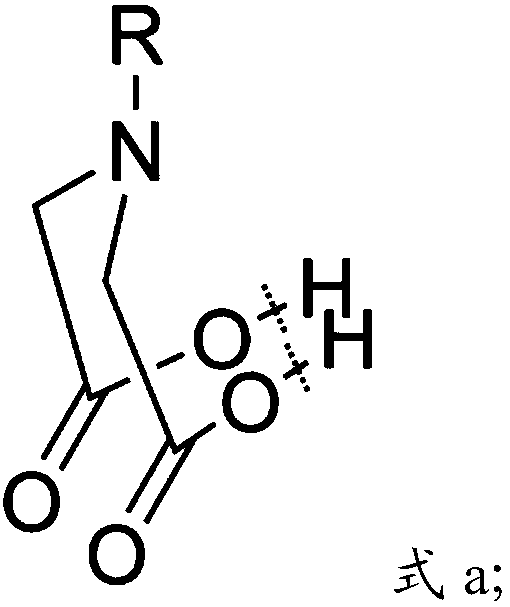
[0110] In the present invention, the iminodiacetic acid derivative preferably has the structure shown in formula a':
[0111]
[0112] Among them, R in formula a' is hydrogen, C1-C18 alkyl, amino, C1-C10 alkylamino, C1-C10 alkoxy, amido, acyloxy, aryl, cyano,...
Embodiment 1
[0156] 1. Preparation of metal complexes
[0157] Dissolve 0.8mmol of N-methyliminodiacetic acid and 1.6mmol of ferric nitrate in 40mL of a mixed solvent of water and methanol (the volume ratio of water and methanol is 1:1), control the temperature at 20°C, and 2 Stir for 10 minutes under the same conditions, then add 1.6 mmol of 4-carboxypyridine, continue to stir for coordination reaction until the color of the solution does not change, filter to obtain a metal complex solution, and crystallize at room temperature to obtain a metal complex;
[0158] 2. Preparation of bleach working solution
[0159] 15 parts by weight of hydrogen peroxide, 0.01 parts by weight of metal complexes, 2 parts by weight of penetrant JFC, 1 part by weight of desizing enzyme F-252, 2 parts by weight of Goon high-capacity scouring enzyme, 0.5 parts by weight of oxygen bleaching stabilizer Goon2010, 1000 parts of water parts by weight.
[0160] 3. Bleaching process
[0161] According to the mass ra...
Embodiment 2
[0163] 1. Preparation of metal complexes
[0164] Dissolve 0.8mmol N-(2-pyridylmethyl)iminodiacetic acid and 0.8mmol cobalt chloride in 40mL water, control the temperature at 40°C, and N 2 Stir for 10 min under the conditions, then add 0.8 mmol of 2,2'-bipyridine, continue to stir for coordination reaction until the color of the solution no longer changes, filter to obtain a metal complex solution, and crystallize at room temperature to obtain a metal complex;
[0165] 2. Preparation of bleach working solution
[0166] 15 parts by weight of hydrogen peroxide, 0.01 parts by weight of metal complexes, 2 parts by weight of fast penetrating agent T, 1.5 parts by weight of Suhong wide temperature desizing enzyme, 2 parts by weight of Goon1013 cold stack scouring enzyme, 0.5 parts by weight of Goon2010 oxygen bleaching stabilizer, water 1000 parts by weight.
[0167] 3. Bleaching process
[0168] According to the mass ratio of textiles and bleaching working solution 1:20, put the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com