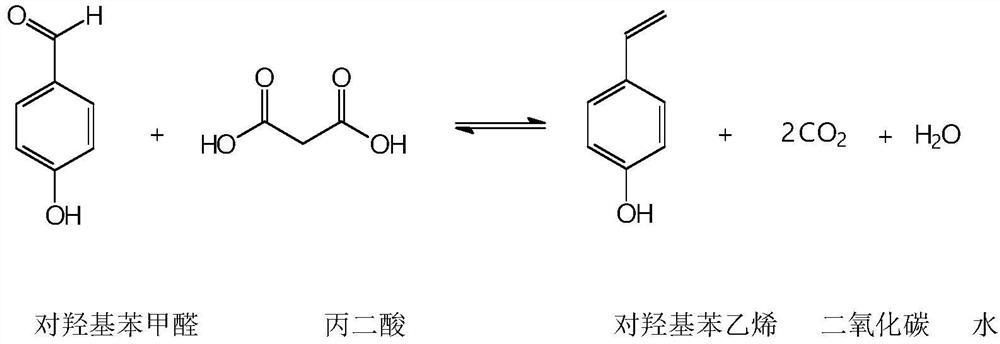
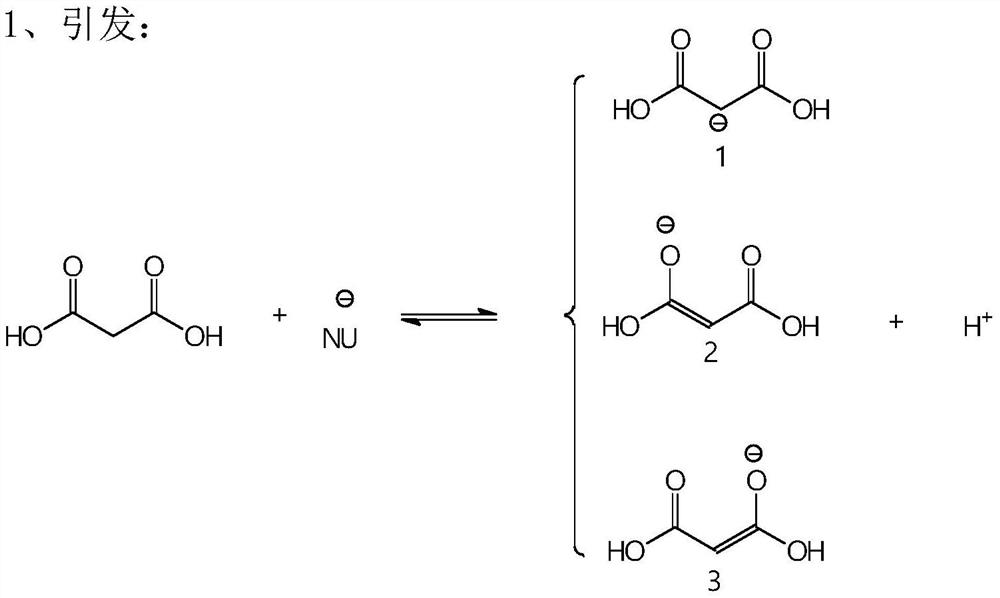
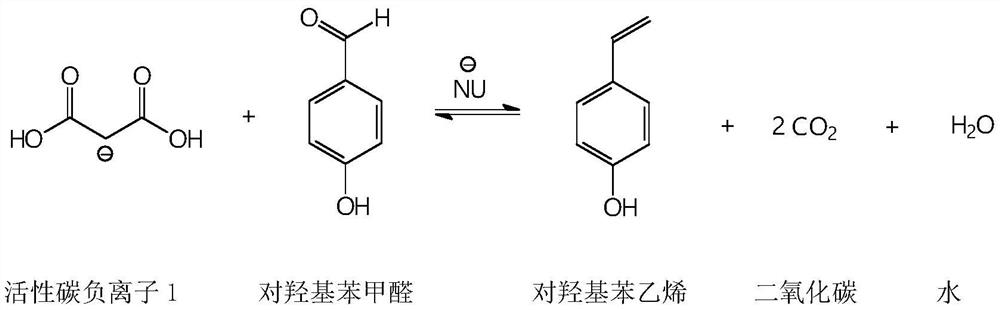
A kind of synthetic method of p-hydroxystyrene
A technology for p-hydroxystyrene and synthesis methods, which is applied in chemical instruments and methods, preparation of organic compounds, separation/purification of carboxylic acid compounds, etc. Improved atom economy, easy solvent recovery and less process waste water
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 50.2 g (0.4069 mol) of p-hydroxybenzaldehyde with a content of 98.9% was dropped into a 250 ml four-necked flask with a magnetic stirring rotor, a thermometer, and a nitrogen-filled tube, and 51.2 g (0.4884 mol) of malonic acid with a content of 99.2% was dropped into. Catalyst copper acetate dihydrate 0.6g, put into 80g of toluene. Fix the three-neck flask on the rectification tower, turn on the nitrogen protection, and control the nitrogen flow rate to 0.5L / min. The rectification column is equipped with glass coil packing with a height of 30cm, which is equivalent to 8-15 theoretical plates.
[0046] The outer wall of the three-necked bottle is heated with an electric heating jacket, and the heating voltage is controlled to about 150V by a voltage stabilizing transformer. Turn on magnetic stirring and slowly heat up. When the internal temperature reaches 120°C, the reaction bottle starts to boil and vaporize. The water formed by the reaction is withdrawn from the t...
Embodiment 2
[0050] 49.8g (0.4037mol) of p-hydroxybenzaldehyde with a content of 98.9% was dropped into a 250ml four-necked flask with a magnetic stirring rotor, a thermometer, and a nitrogen-filled tube, and 51.3g (0.4893mol) of malonic acid with a content of 99.2% was dropped into. Catalyst copper chloride dihydrate 0.7g, put into 80g of toluene. Fix the three-neck flask on the rectification tower, turn on the nitrogen protection, and control the nitrogen flow rate to 0.5L / min. The rectification column is equipped with glass coil packing with a height of 30cm, which is equivalent to 8-15 theoretical plates.
[0051] The outer wall of the three-necked bottle is heated with an electric heating jacket, and the heating voltage is controlled to about 150V by a voltage stabilizing transformer. Turn on magnetic stirring and slowly heat up. When the internal temperature reaches 120°C, the reaction bottle starts to boil and vaporize. The water formed by the reaction is withdrawn from the top o...
Embodiment 3
[0055] 50.0 g (0.4053 mol) of p-hydroxybenzaldehyde with a content of 98.9% was dropped into a 250 ml four-necked flask with a magnetic stirring rotor, a thermometer, and a nitrogen-filled tube, and 51.0 g (0.4865 mol) of a content of 99.2% of malonic acid was dropped into. Catalyst 0.7g of nickel acetate dihydrate was put into 80g of mixed xylenes. Fix the three-neck flask on the rectification tower, turn on the nitrogen protection, and control the nitrogen flow rate to 0.5L / min. The rectification column is equipped with glass coil packing with a height of 30cm, which is equivalent to 8-15 theoretical plates.
[0056] The outer wall of the three-necked bottle is heated with an electric heating jacket, and the heating voltage is controlled by a voltage stabilizing transformer to about 170V. Turn on magnetic stirring and slowly heat up. When the inner temperature reaches 140°C, the reaction bottle starts to boil and vaporize. The water formed by the reaction is withdrawn fro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com