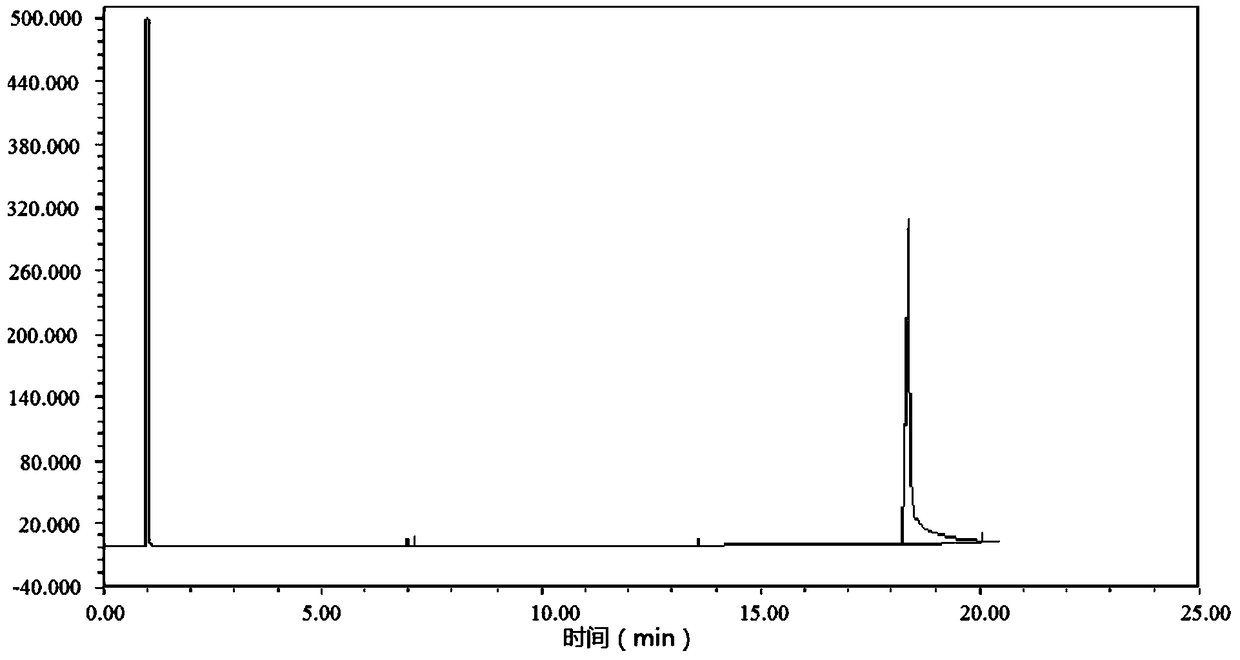
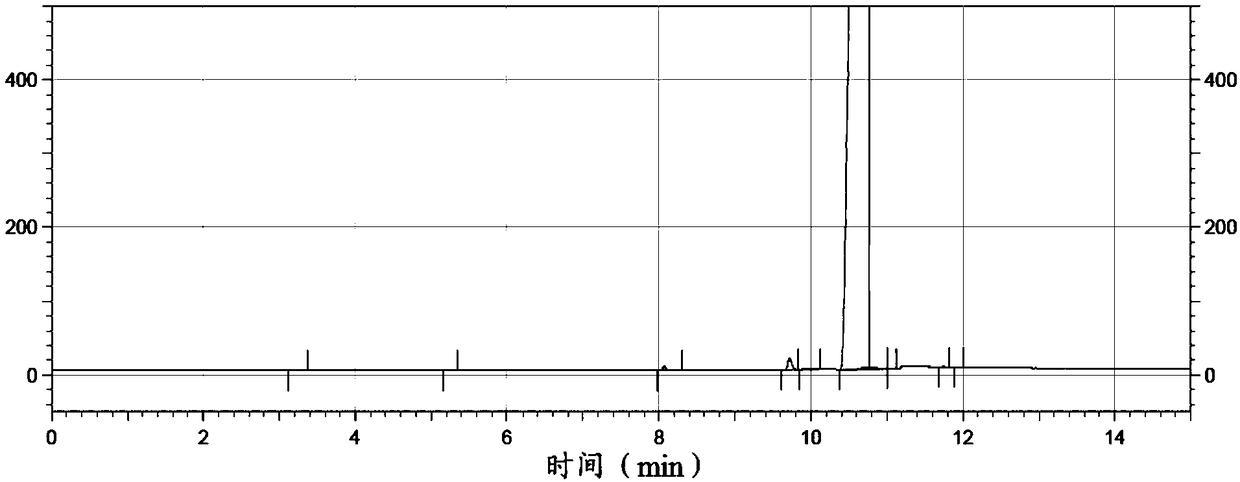
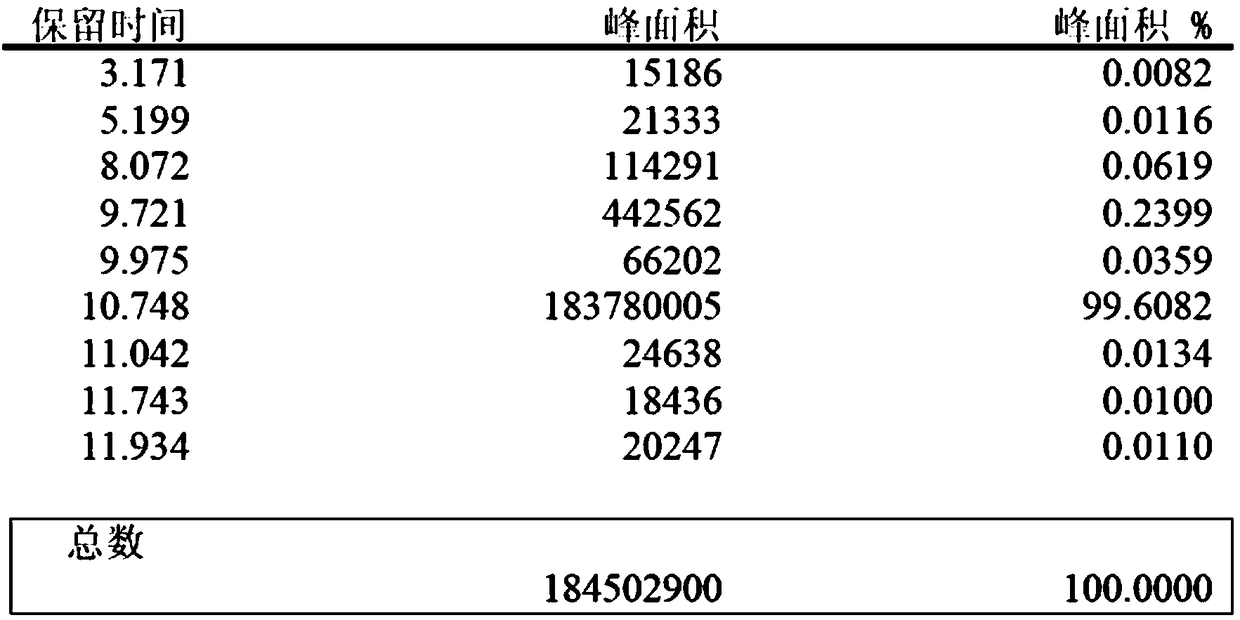
Preparation of methyl p-tert-butylbenzoate
A technology of methyl tert-butyl benzoate and tert-butyl benzoic acid, which is applied in the preparation of carboxylic acid esters, carboxylic acid salts, and organic compounds, and can solve the problem of affecting product purity, high equipment requirements, and dare not incinerate Disposal and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0099] According to a kind of preferred embodiment of the present invention, the aftertreatment of the aqueous phase containing waste product sodium sulfate comprises the following steps:
[0100] Step a, adding inorganic alkali to the aqueous phase containing waste product sodium sulfate;
[0101] In this way, heavy metals (such as Co and Mn) therein will be precipitated and removed under alkaline conditions.
[0102] Step b, add activated carbon to carry out decolorization treatment, then filter and distill water successively to obtain solid sodium sulfate;
[0103] In this way, not only can the colored impurities in sodium sulfate be effectively removed, but also the smell of sodium sulfate can be improved to a certain extent (but activated carbon cannot remove the smell caused by sulfur-containing oligomers), greatly improving the chroma, odor.
[0104] Step c, adding the sodium sulfate solid obtained in step b into an organic solvent, reflux washing, then successively f...
Embodiment 1
[0132] (1) Transfer 500g of p-tert-butyltoluene, 0.968g of cobalt acetate, and 0.032g of manganese acetate into an oxidation kettle, raise the temperature to 125°C, and feed air at a rate of 2200mL / min to carry out the oxidation reaction. During the oxidation reaction process, control the produced water to 24-26g.
[0133] Among them, after the oxidation starts, the heat release in the oxidation process is stable. Although the reaction is stable, the temperature must be lowered, but the temperature reduction is easy to control, and strict cooling control is not required.
[0134] (2) After the oxidation reaction is completed, the material is filtered and transferred to the crystallization kettle, and the temperature is lowered and crystallized at a rate of 6°C / h, centrifuged, and p-tert-butyltoluene is washed to obtain the wet product of p-tert-butylbenzoic acid and the crystallization mother liquor , under a vacuum of 600 Pa, the temperature was raised to 105° C. at a rate of...
Embodiment 2
[0150] (1) 500g of p-tert-butyltoluene, 0.952g of cobalt acetate, and 0.048g of manganese acetate were heated up to 120°C, and air was introduced at a rate of 2000mL / min to carry out the oxidation reaction. During the oxidation reaction process, the amount of produced water is controlled to 24-26g.
[0151] Among them, after the oxidation starts, the heat release in the oxidation process is stable. Although the reaction is stable, the temperature must be lowered, but the temperature reduction is easy to control, and strict cooling control is not required.
[0152] (2) After the oxidation reaction is finished, the material is filtered and transferred to the crystallization tank, and the temperature is lowered and crystallized at a rate of 7°C / h, centrifuged, and p-tert-butyltoluene is washed to obtain the wet product of p-tert-butylbenzoic acid and the crystallization mother liquor , heating up to 110°C at a rate of 15°C / h to dry the wet product of p-tert-butylbenzoic acid to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com