Synthesis method of p-nitrobenzoic acid
A technology of p-nitrobenzoic acid and synthesis method, which is applied in the field of synthesis of p-nitrobenzoic acid, can solve the problems of low product yield and purity, low reaction safety, and high equipment requirements, and achieve short reaction routes and high product purity. High effect with low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Preparation of 2-p-nitrobenzoyl-2-acetamido-1,3-propanediol
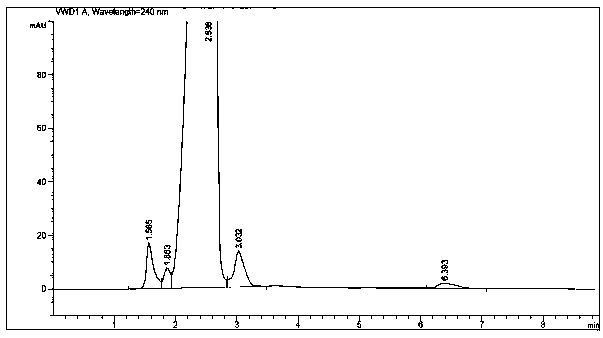
[0023] The method is: first add 400kg of methanol, 420kg of 2-acetamido 4-nitroacetophenone, stir for 30 minutes, add dropwise 200kg of formaldehyde with a mass percentage of 37%, then add 0.5kg of sodium bicarbonate, and slowly heat to 35°C. Stop heating and control the reaction within 45°C, control the reaction sampling, cool to 5°C after the reaction is completed, centrifuge and filter to obtain 380kg of chloramphenicol intermediate, and then carry out vacuum distillation on the centrifuged mother liquor to recover methanol; after cooling to 10°C, centrifuge Promptly get 40kg of 2-p-nitrophenyl 2-acetylaminopropanediol product, and the mass percentage composition is 98.07%, and its content HPLC detection result is as follows figure 1 shown.
Embodiment 2
[0025] The synthetic method of p-nitrobenzoic acid:
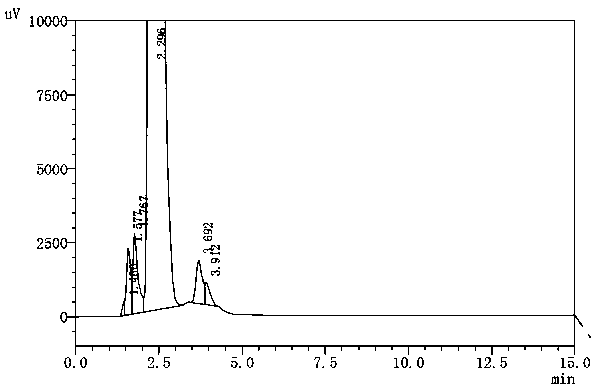
[0026] Add 50 g of 2-p-nitrobenzoyl-2-acetylamino-1,3-propanediol prepared in Example 1 into a 500 ml three-neck flask, then add 190 g of water and 60 g of nitric acid, and slowly heat up to 100°C for reflux reaction 6 Hour, then cool to 20 ℃ of crystallizations with cooling water, filter to obtain p-nitrobenzoic acid 28.5 grams, yield 96.1%, content 99.5%, its content HPLC detection result is as follows: figure 2 shown.
Embodiment 3
[0028] The synthetic method of p-nitrobenzoic acid:
[0029] Put 50 g of 2-p-nitrobenzoyl-2-acetylamino-1,3-propanediol prepared in Example 1 into a 500 ml three-necked flask, then add 200 g of water and 65 g of nitric acid, and slowly heat up to 100°C for reflux reaction 6 Hours, then cooled to 20 ° C crystallization with cooling water, filtered to obtain 29 grams of p-nitrobenzoic acid, yield 97.8%, content 99.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com