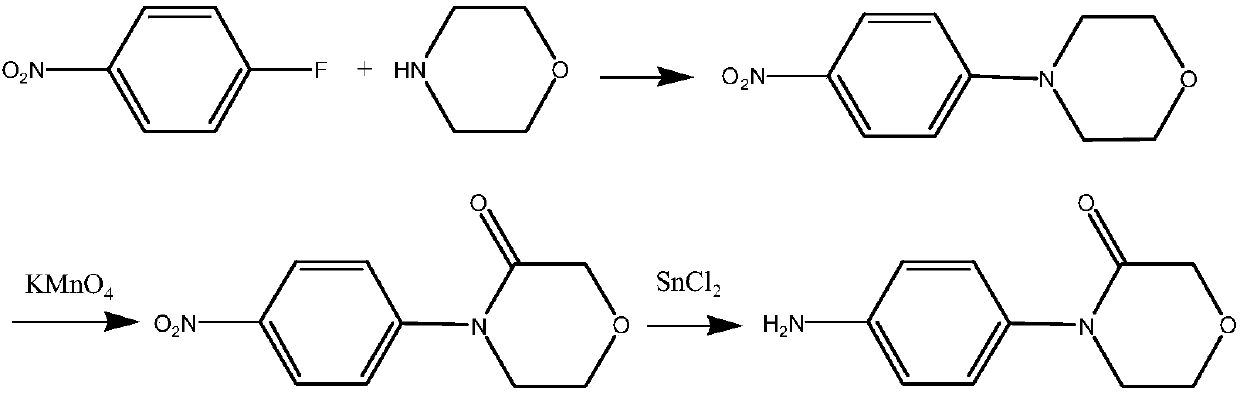
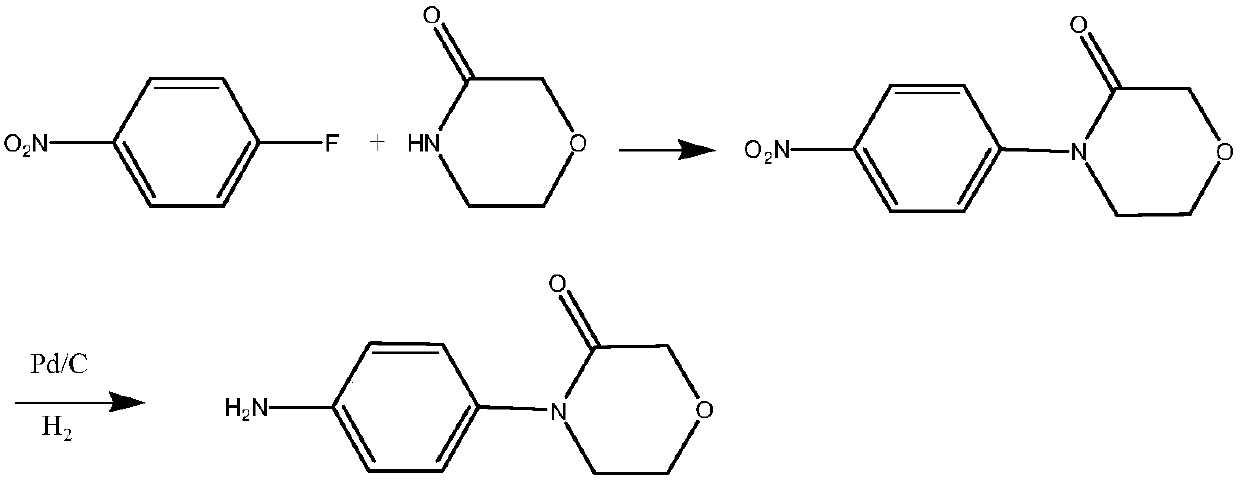
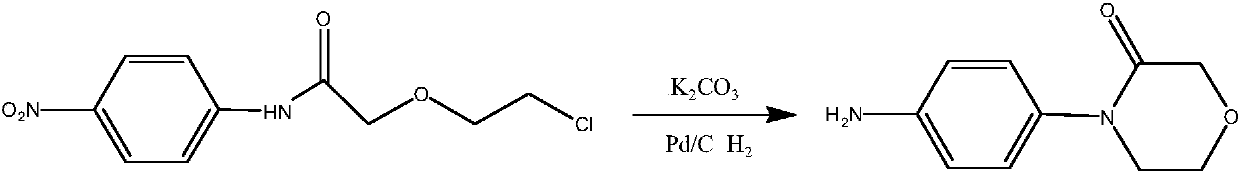
A kind of preparation method of 4-(4-aminophenyl)-3-morpholinone
A technology of aminophenyl and morpholinone, which is applied in the field of preparation of 4--3-morpholinone, can solve the problems of being unsuitable for large-scale industrial production, difficult amidation reaction, and unsuitable for large-scale industrial production, etc. Good quality, improved reaction efficiency, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] The present embodiment prepares 4-(4-aminophenyl)-3-morpholinone through the following steps
[0069] (1) Mix 1 mole of 2-bromoethylamine hydrobromide with 500 ml of acetonitrile, cool in an ice-water bath to 10°C, add 1 mole of anhydrous potassium carbonate in batches under nitrogen protection, and control the temperature of the system not higher than 20°C, after the addition is complete, stir for 30 minutes to remove potassium bromide by filtration, then add 1 mole of anhydrous potassium carbonate, and add 1.2 moles of p-fluoronitrobenzene dropwise with stirring at room temperature. After dropping, the temperature was raised to 45°C for condensation reaction for 8h. GC detected that the reaction of 2-bromoethylamine was complete. The acetonitrile solvent was concentrated and distilled to dryness under reduced pressure. Add 300 ml of ethyl acetate and 200 ml of distilled water, stir for 15 minutes and then separate the layers. The aqueous phase was discarded, and th...
Embodiment 2
[0077] The present embodiment prepares 4-(4-aminophenyl)-3-morpholinone through the following steps
[0078] (1) Mix 1 mole of 2-chloroethylamine hydrogen hydrochloride with 500 milliliters of acetonitrile, cool in an ice-water bath to 10°C, add 1 mole mass of anhydrous cesium carbonate in batches under nitrogen protection, and control the temperature of the system not higher than 20°C, after the addition is complete, stir for 30 minutes to remove cesium chloride by filtration, then add 1 mole of anhydrous cesium carbonate, and add 2 moles of p-fluoronitrobenzene dropwise with stirring at room temperature. After dropping, heat up to 80°C for condensation reaction for 5h. GC detected that the reaction of 2-chloroethylamine was complete. The acetonitrile solvent was concentrated and distilled to dryness under reduced pressure. Add 300 ml of ethyl acetate and 200 ml of distilled water, stir for 15 minutes and then separate the layers. The aqueous phase was discarded, and the o...
Embodiment 3
[0086] The present embodiment prepares 4-(4-aminophenyl)-3-morpholinone through the following steps
[0087] (1) Mix 1 mole of 2-bromoethylamine hydrobromide with 500 ml of acetonitrile, cool in an ice-water bath to 10°C, add 1 mole of anhydrous potassium carbonate in batches under nitrogen protection, and control the temperature of the system not higher than 20°C, after the addition is complete, stir for 50 minutes to remove potassium bromide by filtration, then add 1.5 moles of anhydrous potassium carbonate, and add 0.5 moles of p-fluoronitrobenzene dropwise with stirring at room temperature. After dropping, heat up to 35°C for condensation reaction for 10h. GC detected that the reaction of 2-bromoethylamine was complete. The acetonitrile solvent was concentrated and distilled to dryness under reduced pressure. Add 250 ml of ethyl acetate and 180 ml of distilled water, stir for 10 minutes and then separate the layers. The aqueous phase was discarded, and the organic phase...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com