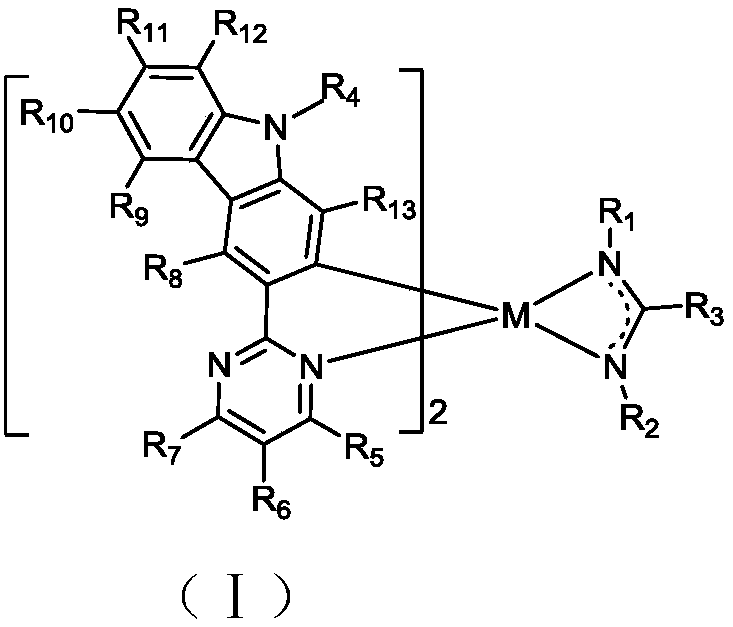
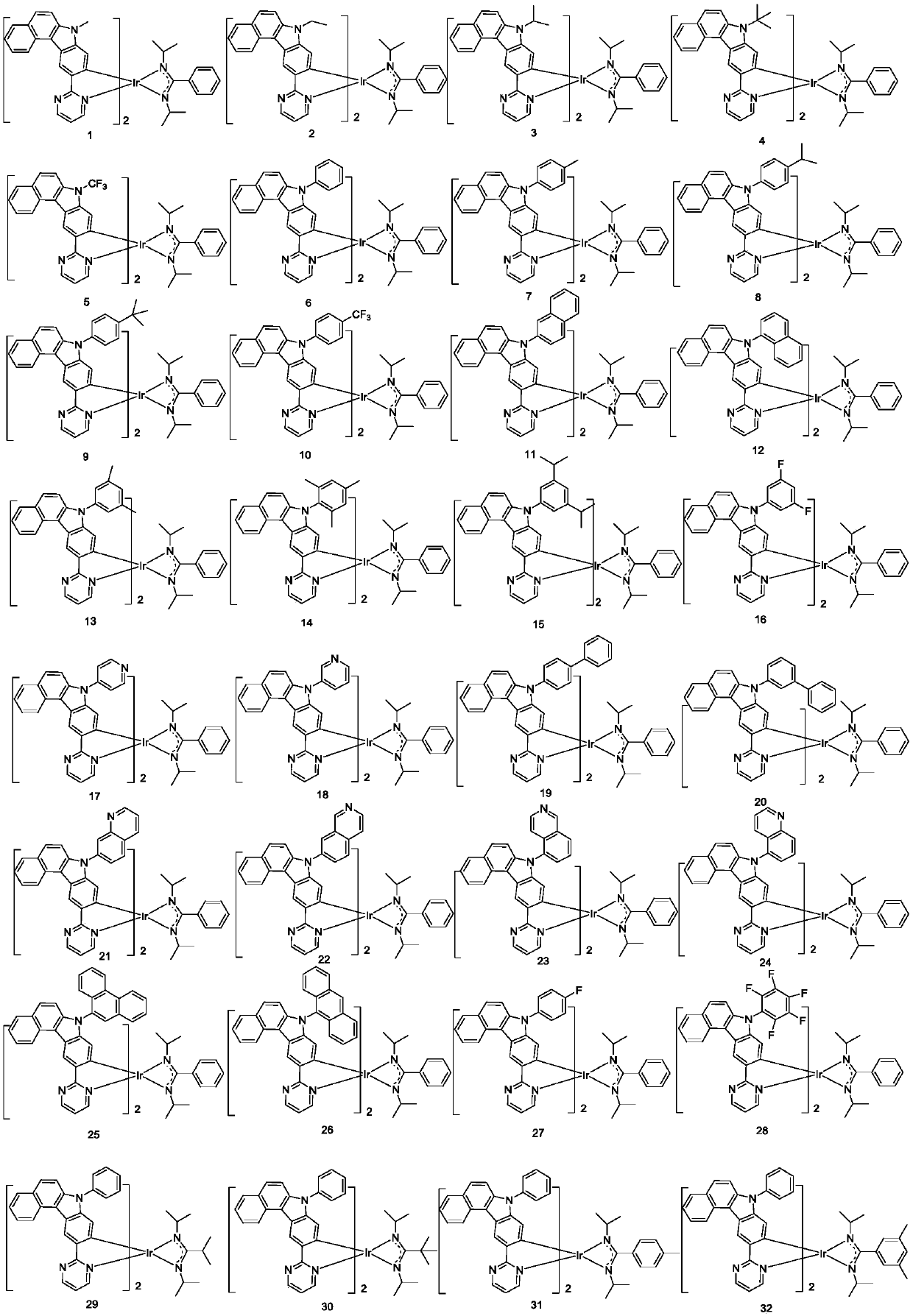
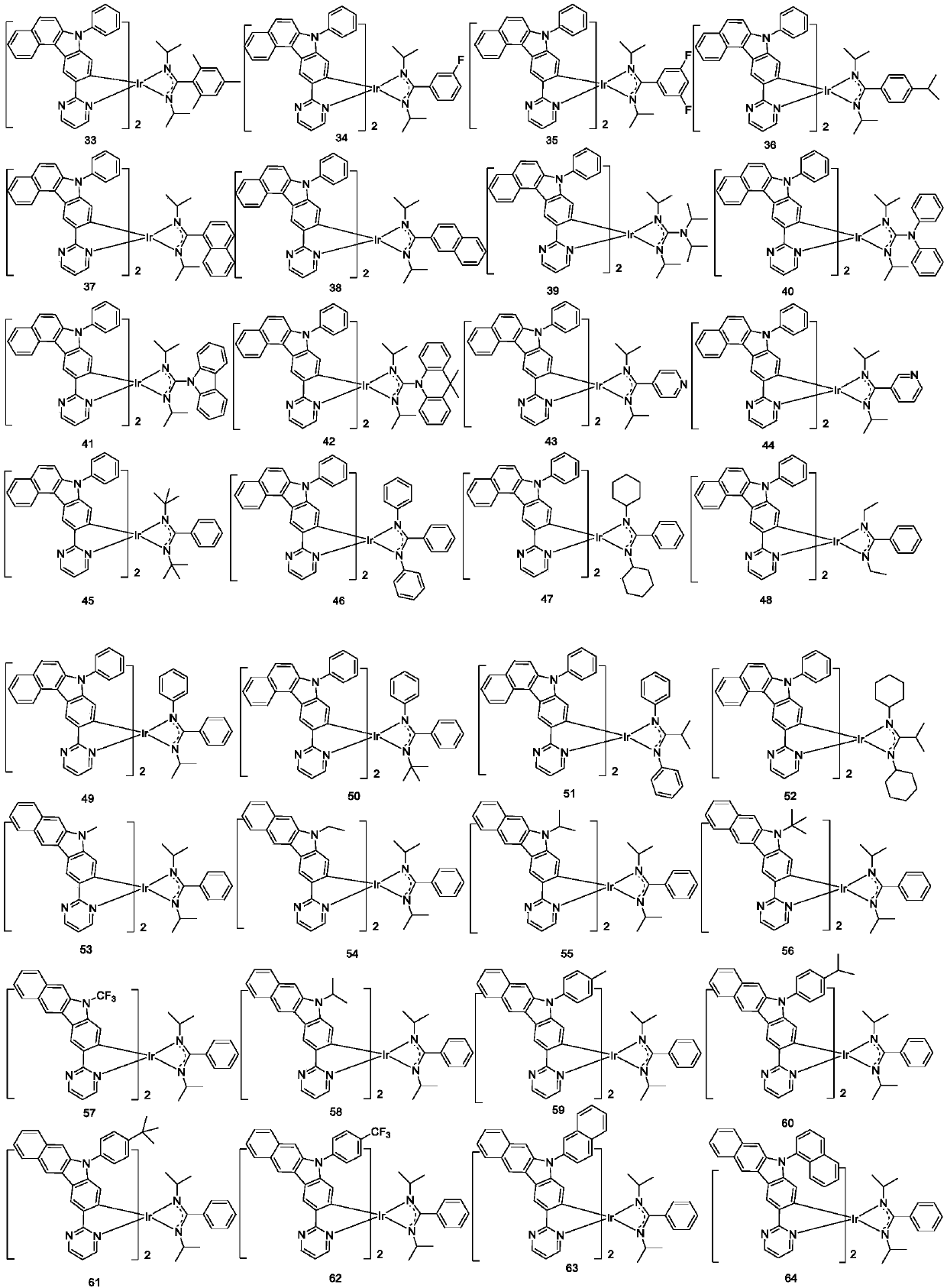
Complex and OLED (organic light emitting device)
A technology of organic light-emitting devices and complexes, which is applied in the direction of light-emitting materials, organic chemistry, and electric solid-state devices. It can solve the problems of poor thermal stability, low luminous efficiency, and high driving voltage, and achieve low driving voltage and high luminous efficiency. The effect of production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1: Preparation of compound 3
[0077]
[0078] Preparation of intermediate A3
[0079] Take compound b3 (5.92g, 20mmol), isopropyl iodide (3.40g, 20mmol), activated copper powder (2.56g, 40mmol), sodium hydroxide (1.6g, 40mmol), 140ml of xylene, and reflux for 24h under nitrogen atmosphere . After filtering, the filtrate is decompressed to recover the solvent and recrystallized with ethanol to obtain compound c3.
[0080] The compound c3 (7.34 g, 21.7 mmol), 50 ml of tetrahydrofuran, and nitrogen atmosphere were cooled to -75° C.-85° C., n-butyl lithium (18 ml, 36 mmol) was added dropwise, and the reaction was incubated for 1 h. Tributyl borate (10 g, 43.4 mmol) was added dropwise, and the temperature began to rise after the reaction was kept for 1 hour. After the reaction system rises to 0°C, the reaction solution is slowly poured into the vigorously stirred cold dilute hydrochloric acid, filtered and drained, and dried under vacuum to obtain a white compound d3.
...
Embodiment 2
[0088] Example 2: Preparation of Compound 6
[0089] The isopropyl iodide in Example 1 was replaced with equimolar a6, and the other steps were the same as the synthesis of Example 1, to obtain the target compound 6 (6.60 g, 29%).
[0090]
[0091] Mass spectrum m / z: 1137.40 (calculated value: 1137.39). Theoretical element content (%) C 65 H 52 IrN 8 : C, 68.64; H, 4.61; Ir, 16.90; N, 9.85 Measured element content (%): C, 68.65; H, 4.60; Ir, 16.90; N, 9.85. The above results confirm that the obtained product is the target product.
Embodiment 3
[0092] Example 3: Preparation of compound 11
[0093] The isopropyl iodide in Example 1 was replaced with equimolar a11, and the other steps were the same as the synthesis of Example 1, to obtain the target compound 11 (7.17 g, 29%).
[0094]
[0095] Mass spectrum m / z: 1237.44 (calculated value: 1237.43). Theoretical element content (%) C 73 H 56 IrN 8 : C, 70.85; H, 4.56; Ir, 15.53; N, 9.05 Measured element content (%): C, 70.86; H, 4.55; Ir, 15.53; N, 9.05. The above results confirm that the obtained product is the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com