Pharmaceutical composition containing chemical ablating agent and vaccine adjuvant, and application thereof
A vaccine adjuvant and composition technology, which can be applied in the directions of drug combinations, active ingredients of heterocyclic compounds, and medical preparations containing active ingredients, etc., can solve the problems of high recurrence rate and low curative effect of intractable diseases related to local lesions, etc., Achieving the effect of low cost, small systemic side effects, and rapid reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0188] Embodiment 1: the preparation of composition
[0189] 1. Preparation of liquid preparations
[0190] Using the aforementioned preparation method, the composition of the part of the pharmaceutical composition solution of the present invention prepared in this embodiment is listed in Table 2. The excipients therein include vehicles, and unless otherwise specified, all vehicles contain water for injection. The pharmaceutical composition solutions are all stored for more than 2 hours after preparation and used again.
[0191] Table 2
[0192]
[0193]
[0194]*: The vehicle is an aqueous solution containing 25% ethanol, 15% PEG300 and 15% propylene glycol.
[0195] Specifically, for example, based on the final 100ml composition solution volume, at room temperature, measure the corresponding chemical ablative agent (such as methylene blue or / and quinine dihydrochloride) and aluminum hydroxide according to the amount shown in Table 2 Add the dry powder of microparti...
Embodiment 2
[0204] Example 2: Synergy Research
[0205] 1. Research on collaborative conditions
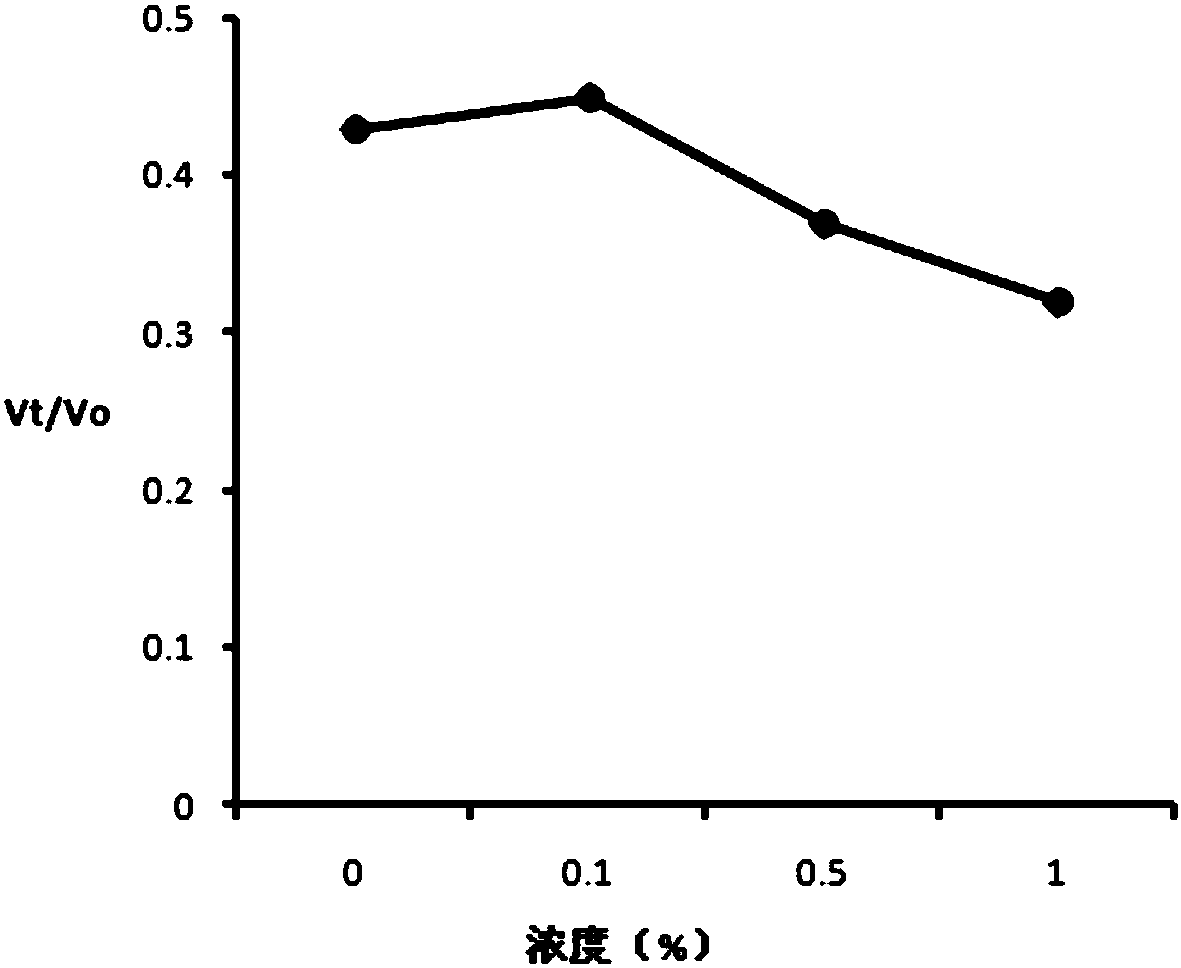
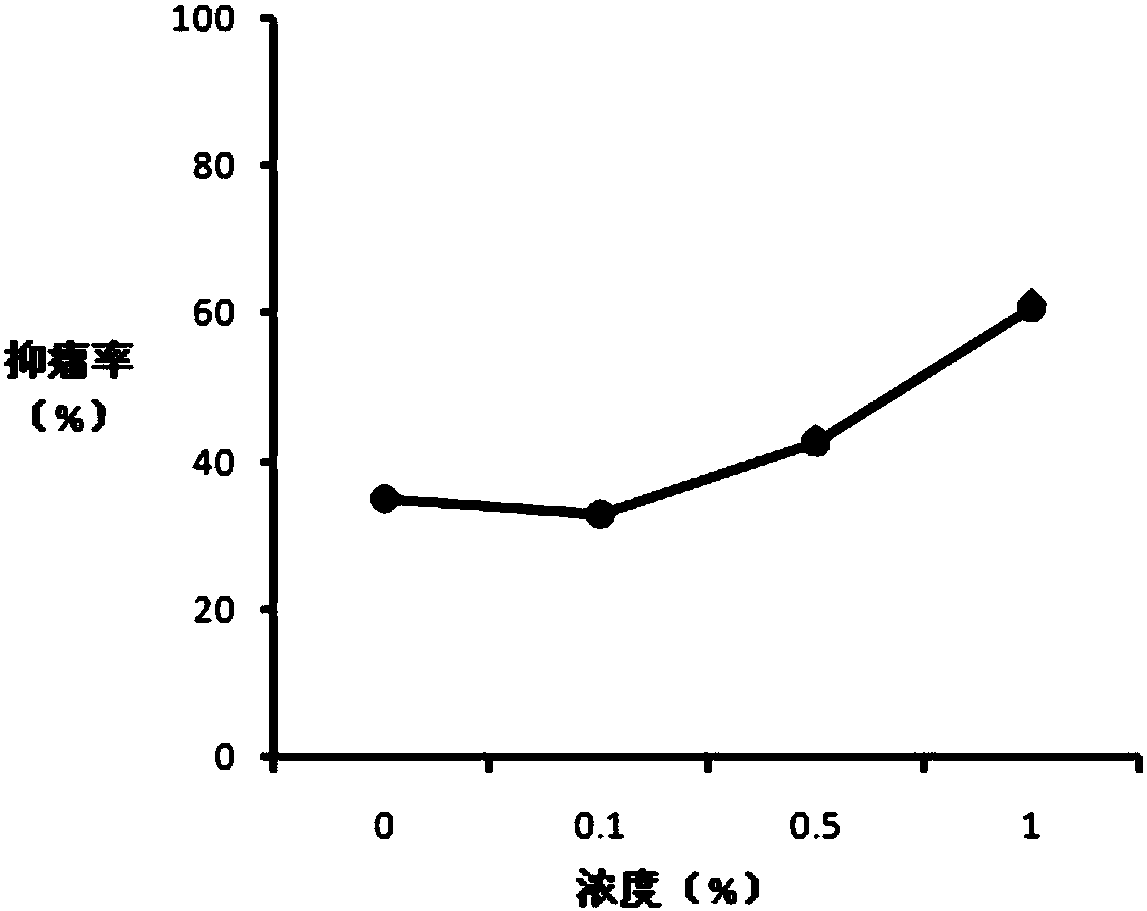
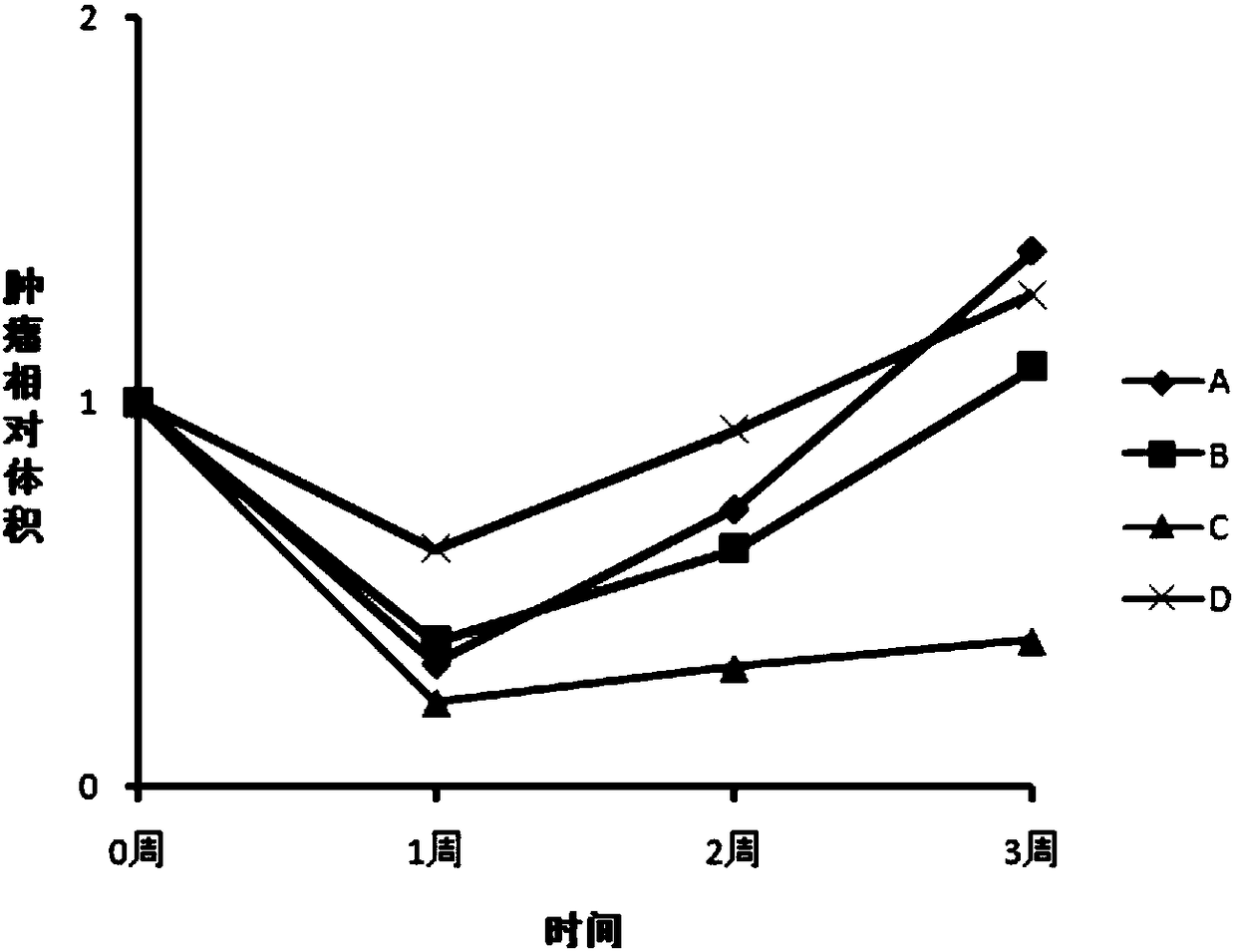
[0206] One for mice bearing S180 cells (118mm 3 ) experiments, the successfully modeled test animals were randomly divided into negative control group and research group. The negative control group was intratumorally injected with 100 μl of normal saline, and the four study groups (groups A, B, C, and D) were injected intratumorally with 5 methylene blue / aluminum hydroxide particle compositions. In the composition, the concentration of methylene blue is 2%, and the concentration of aluminum hydroxide particles is 0%, 0.1%, 0.5%, and 1%, respectively. The research drugs are all suspensions prepared according to the preparation method of Example 1 with water for injection as the vehicle. The dose of methylene blue is 100mg / kg animal. Medication was administered once, and the animals were euthanized and dissected 21 days after the administration. Figure 1 (1-1 and 1-2) shows the effect of c...
Embodiment 3
[0227] Embodiment 3: super effective activity research
[0228] Activity is actually a relative concept. Within the scope of the present invention, the term "activity" refers to the property of a substance showing a certain therapeutic effect, the term "effective activity" refers to the property of a substance having a therapeutic effect equivalent to existing clinical drugs, and the term "super-effective activity" "Refers to the property that a substance has significantly higher therapeutic effects compared with existing clinical drugs. Taking existing clinical anti-tumor chemotherapeutic drugs (such as 5-fluorouracil) as an example, their tumor inhibition rate in the tumor-bearing mouse test is 45-75%. Taking existing clinical anti-tumor immune drugs (such as autologous tumor vaccines) as an example, their tumor-inhibitory rate in the tumor-bearing mouse test is 45-80%. This is the curative effect of existing clinical medicine. Hyperpotent activity requires significantly ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com