Organic-inorganic composite electrolyte and preparation method thereof
A composite electrolyte and inorganic composite technology, applied in circuits, fuel cells, electrical components, etc., can solve the problems of slow electrode reaction, complex battery structure, and large energy consumption, and achieve good medium-temperature proton conductivity, simple process and operation, The effect of large output power density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] (1) Sn 0.85 Ga 0.15 P 2 o 7 preparation of
[0068] Weigh 0.85mol of tin oxide, 0.15mol of gallium oxide and 2mol of concentrated phosphoric acid, grind and mix them, mix them evenly and heat them at 200-300°C until they become a paste, then continue stirring at 350°C until they are fully reacted and become powder, then pulverize. Ball milled for 8 hours, and the mixture was dried and sieved;
[0069] The above system was sintered at 600°C for 2h to obtain Sn 0.85 Ga 0.15 P 2 o 7 (denoted as SGPO) samples, with the single Sn 0.85 Ga 0.15 P 2 o 7 The fuel cell assembled as electrolyte has a conductivity of 8.1×10 at 300°C -3 S cm -1 , the output power density is 28.6mW·cm -2 .
[0070] (2) Preparation of composite electrolyte
[0071] Composite polyphenylene ether (PPO) dissolved in chloroform with ball-milled and sieved SGPO at a ratio of 4:1, stir for 0.5-1 hour, shake, and evaporate until viscous;
[0072] The system was laid on the surface of the sil...
Embodiment 2
[0075] The method used in this embodiment is similar to the method used in Example 1, the only difference being that the consumption of raw materials in (1) is respectively 0.95mol tin oxide, 0.05mol gallium oxide and 2mol concentrated phosphoric acid. 0.95 Ga 0.05 P 2 o 7 The fuel cell assembled as electrolyte has a conductivity of 6.7×10 at 300°C -3 S cm -1 , the output power density is 20.1mW·cm -2 .
[0076] A fuel cell assembled with the composite electrolyte prepared in this example has an electrical conductivity of 1.02×10 in a humid oxygen atmosphere at 300° C. -2 S cm -1 , the output power density is 50.3mW·cm -2 .
experiment example 1
[0078] XRD characterization of experimental example 1 sample
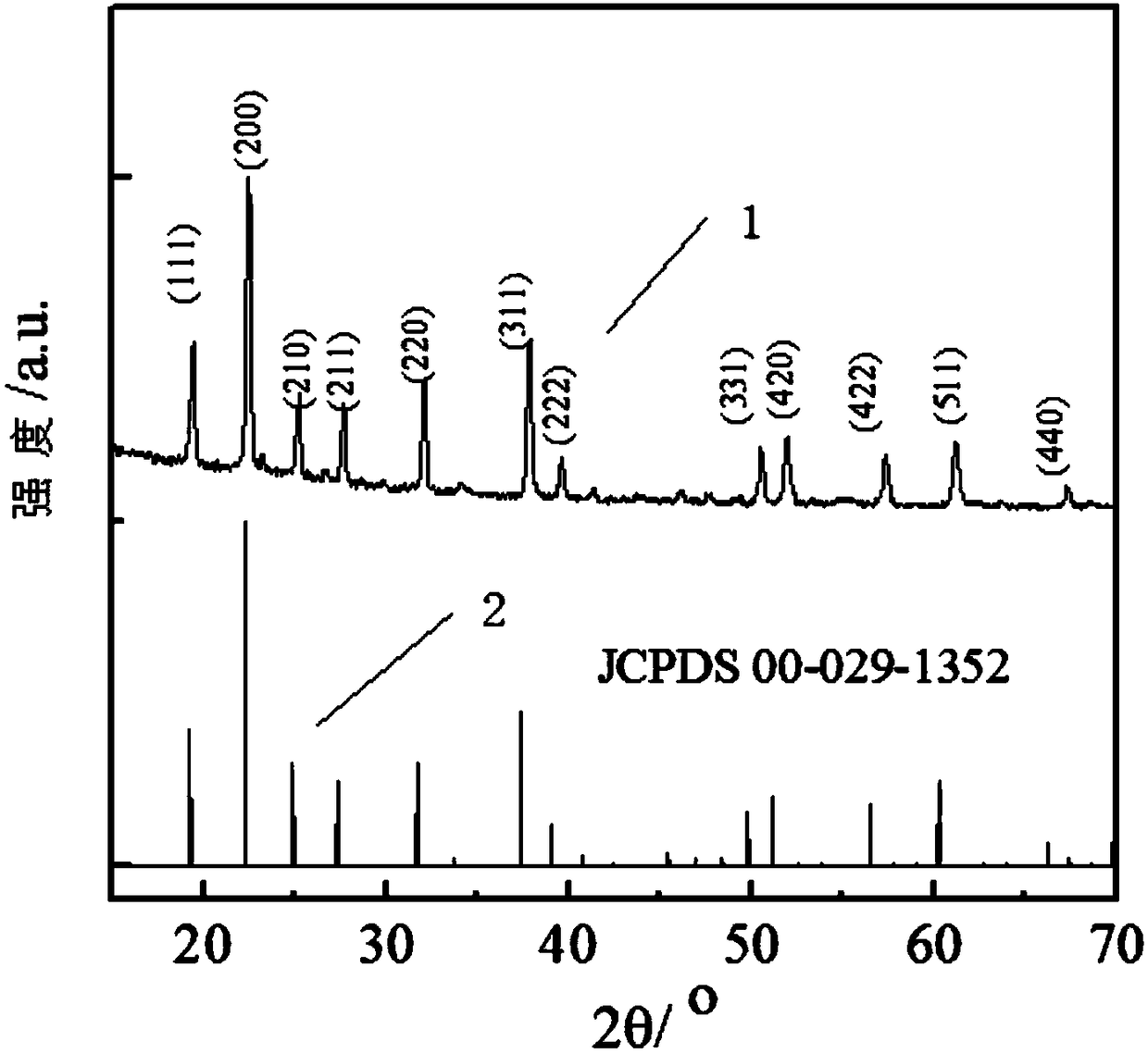
[0079] With the phase structure of the sample that XRD instrument test embodiment 1 makes, the result is as figure 1 As shown, among them,
[0080] Curve 1 shows the XRD collection of illustrative plates that embodiment 1 makes composite electrolyte;
[0081] Curve 2 shows SnP 2 o 7 (JCPDS 00-029-1352) standard XRD pattern;
[0082] Depend on figure 1 It can be seen that the XRD of the composite electrolyte sample prepared in Example 1 has (111), (200), (210), (211) respectively at 2θ of 19.2°, 22.2°, 25.0°, 27.4°, 31.8° and 37.4° , (220) and (311) crystal plane diffraction peaks, crystal plane index and cubic phase SnP 2 o 7 (JCPDS 00-029-1352), no new diffraction peaks were produced in the figure after compounding with polyphenylene ether (PPO), indicating that no new substance was formed after the compounding of the two, that is, gallium-doped tin pyrophosphate and poly There is no chemical reaction in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com