Polypeptide for prolonging blood circulation time of bacteriophage
A technology of blood circulation time and bacteriophage, which is applied in the field of biomedicine to achieve the effect of prolonging blood circulation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
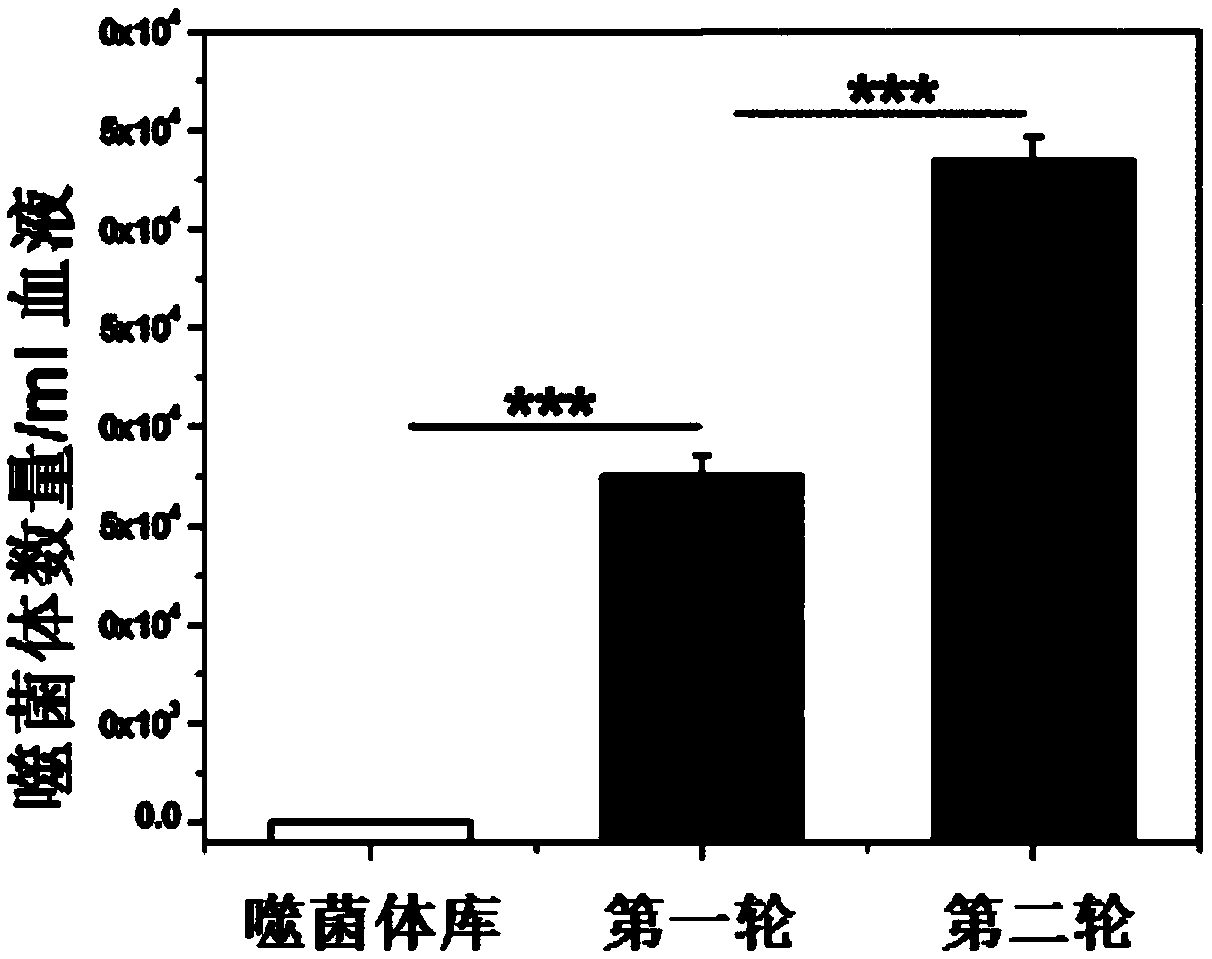
[0053] Example 1: Screening of specific time-delayed short peptides
[0054] In this example, the library used to screen specific time-delayed short peptides is provided by New England Biolabs, which is a heptapeptide library containing disulfide bonds (Ph.D. TM -C7C). Random fragments in this library are flanked by cysteine residues that can be oxidized during phage assembly to form disulfide linkages, thus forming a cyclic peptide that interacts with the target. This library contains more than two billion clones. Random peptides in the library are amino-terminal to the small coat protein pIII, so five copies are expressed per phage particle. Ph.D. TM The position of the phage-expressed random sequence in the -C7C library is preceded by alanine-cysteine. There is a short linker sequence (glycine-glycine-glycine-serine) between the random peptide and the pIII protein. (Ph.D. TM - C7C Phage Peptide Display Kit, http: / / www.neb.com / nebecomm / products / productE8120.asp).
...
Embodiment 2
[0061] Example 2: Delay Capability Test
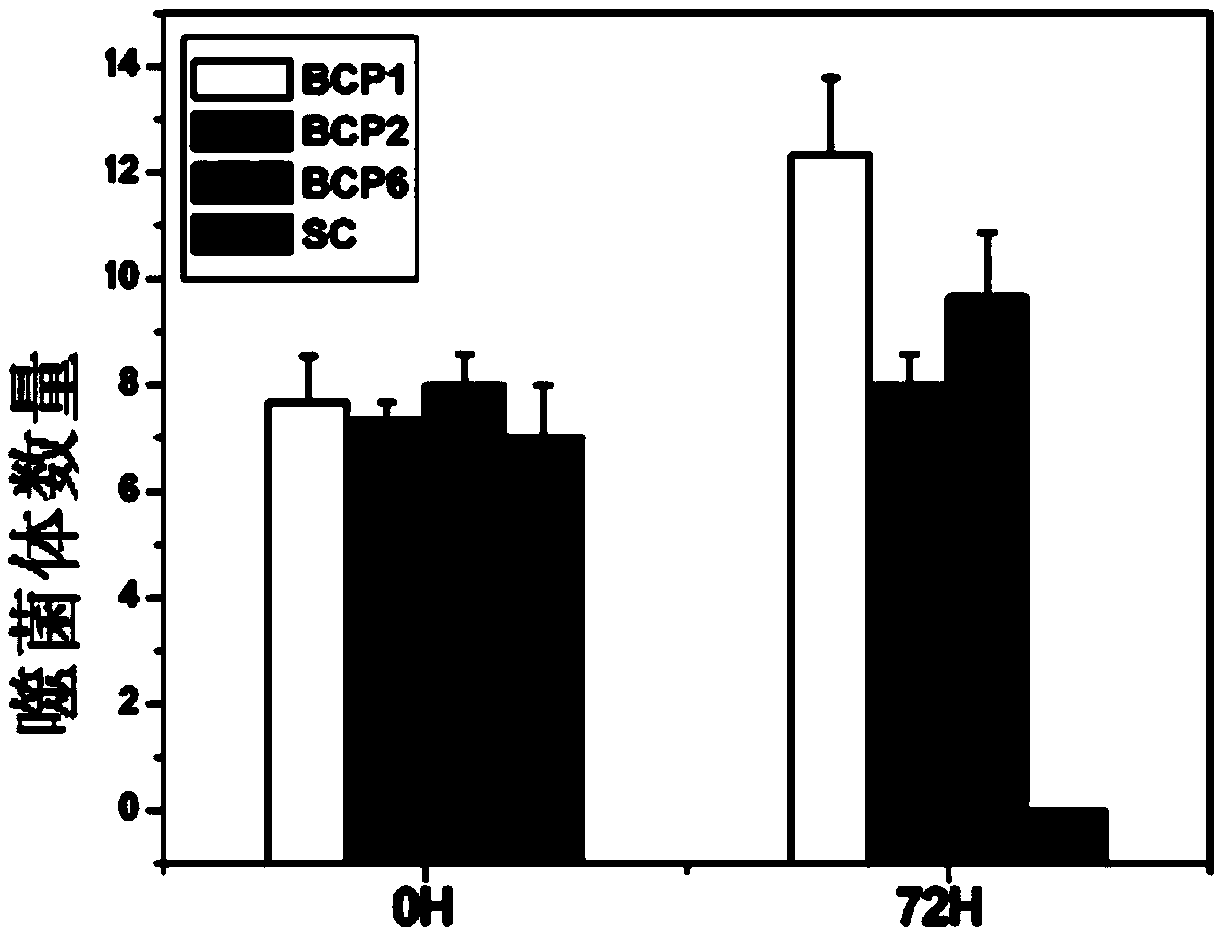
[0062] Take 1×10 respectively 11 A phage carrying the displayed peptides BCP1, BCP2, and BCP6 obtained in Example 1 was mixed with random phage SC (displayed peptide sequence: CNATLPHQC, SEQ ID NO: 4), and injected into the rat body through the tail vein, respectively, at 0h (3min). ) and 72h to take blood plate to detect phage.
[0063] figure 2 The number of phages at 0h and 72h are shown. It can be seen from the figure that the numbers of the four phages are similar at 0 h, but the SC phages have disappeared at 72 h, and the proportion of BCP1 is the highest, followed by BCP6 and BCP2. It can be seen that the phages carrying the three displayed peptides obtained in Example 1 all have the ability to significantly prolong the circulation time of the phages in the blood of rats.
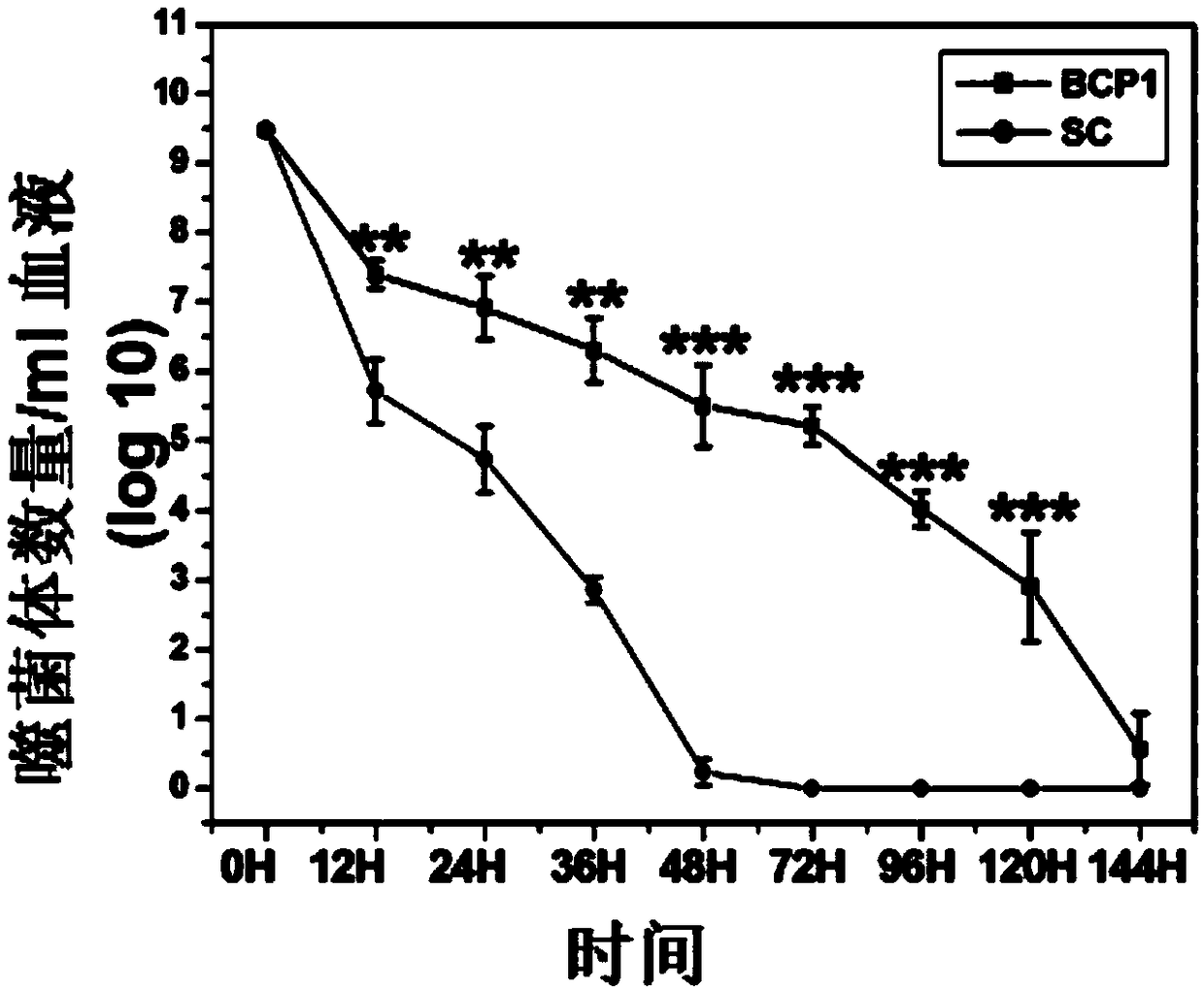
[0064] In order to further confirm the time-delay effect of BCP1, we injected equal amounts of BCP1 and SC phages into rats respectively, and detected t...
Embodiment 3
[0068] Example 3: Verification that the time-delay function of phage has sequence specificity
[0069]During the screening process, we found that as the phages were enriched round after round, the frequency of phages containing RGD sequences increased continuously. Therefore, in order to determine the importance of RGD in the phage display peptide sequences obtained in Example 1, we constructed Three BCP1 phages were identified, as follows:
[0070] TB1: CRNHDMGAC (SEQ ID NO: 6, disrupted BCP1 sequence);
[0071] TB2: CNAAGAMHC (SEQ ID NO: 7, mutated RGD to AGA);
[0072] TB3: CAARGDAAC (SEQ ID NO: 8, RGD retained, remaining four amino acids mutated to A).
[0073] The 1×10 11 A TB1, TB2, TB3, BCP1, and SC phage were injected into different rats respectively, or mixed with REW 1:1 and injected into the same rat, and the number of phages in the blood at different time points was detected, and the results were as follows: Figure 5 shown.
[0074] Figure 5 The results sho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com