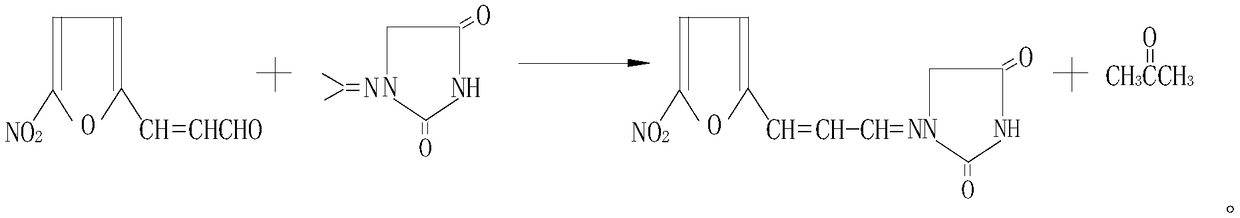
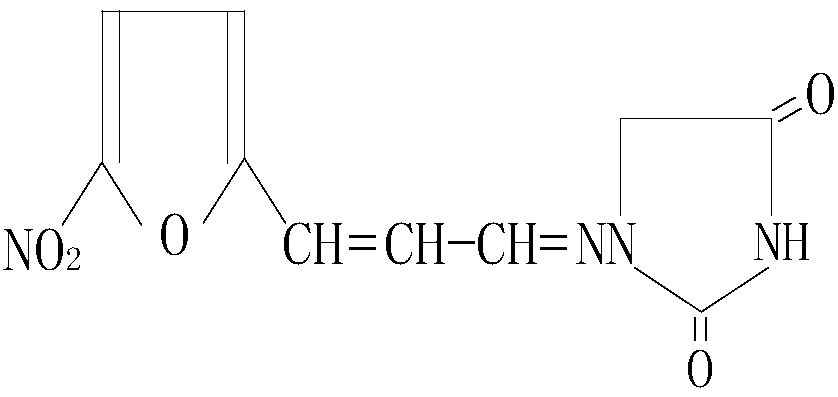
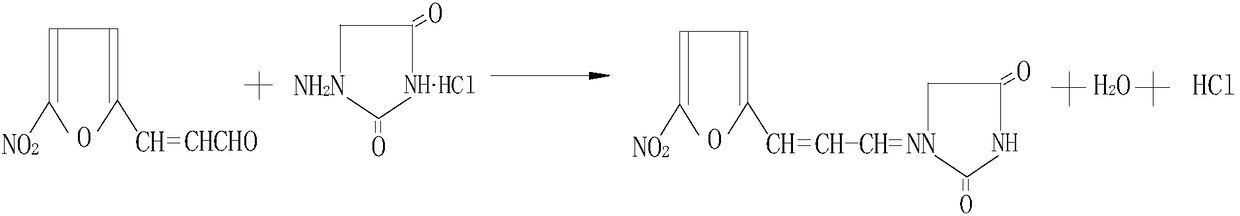
Synthesis method of 1-((3-(5-nitro-2-furyl)allylidene)amino) hydantoin
A technology of nitrofuran acrolein and allylidene, which is applied in the direction of organic chemistry and the like, can solve the problems of aggravating environmental impact pressure, poor atom economy, increasing production cost, etc., and achieves improved atom economy, less three wastes, and reduced generation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] In a 3000L reactor, add 2400kg of 5% propylaminohydantoin solution, 150kg of 5-nitrofuran acrolein and 400kg of N,N-dimethylformamide (DMF) in sequence, and heat to 90~ React at 100°C for 1.5 hours, cool to room temperature, add 400kg of water to dilute and precipitate a brown-yellow solid, filter, wash with water, wash with ethanol and dry to obtain a brown-yellow powder, which is then dissolved in N,N-dimethylformamide, decolorized, and refined , washing with ethanol to obtain orange powder, drying to obtain target product 1-((3-(5-nitro-2-furyl) allyl) amino) hydantoin 180kg, yield 80%, also obtain propylene base amino solid.
Embodiment 2
[0045] In a 3000L reactor, add 2400kg of 5% propylaminohydantoin solution, 160kg of 5-nitrofuran acrolein and 400kg of N,N-dimethylformamide (DMF) in sequence, and heat to 90~ React for 1 hour at 100°C, cool to room temperature, add 400kg of water to dilute and precipitate a brownish-yellow solid, filter, wash with water, wash with ethanol and dry to obtain a brownish-yellow powder, which is then dissolved in N,N-dimethylformamide, decolorized, and refined , washing with ethanol to obtain orange-yellow powder, drying to obtain the target product 1-((3-(5-nitro-2-furyl) allylidene) amino) hydantoin 175kg, yield 79%, also obtain propylene base amino solid.
Embodiment 3
[0047]In a 3000L reactor, add 2400kg of 5% propylaminohydantoin solution, 160kg of 5-nitrofuran acrolein and 400kg of N,N-dimethylformamide (DMF) in sequence, and heat to 90~ React at 100°C for 2 hours, cool to room temperature, add 400kg of water to dilute and precipitate a brown-yellow solid, filter, wash with water, wash with ethanol and dry to obtain a brown-yellow powder, which is then dissolved in N,N-dimethylformamide, decolorized, and refined , washing with ethanol to obtain orange powder, drying to obtain the target product 1-((3-(5-nitro-2-furyl) allylidene) amino) hydantoin 185kg, yield 83%, HPLC analysis purity The ratio was 99.5%, and a solid of propyl amino was obtained.
[0048] The 1-((3-(5-nitro-2-furyl)allylidene)amino)hydantoin compound prepared in Examples 1-3 was verified by ultraviolet, infrared, nuclear magnetic resonance, mass spectrometry and elemental analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com