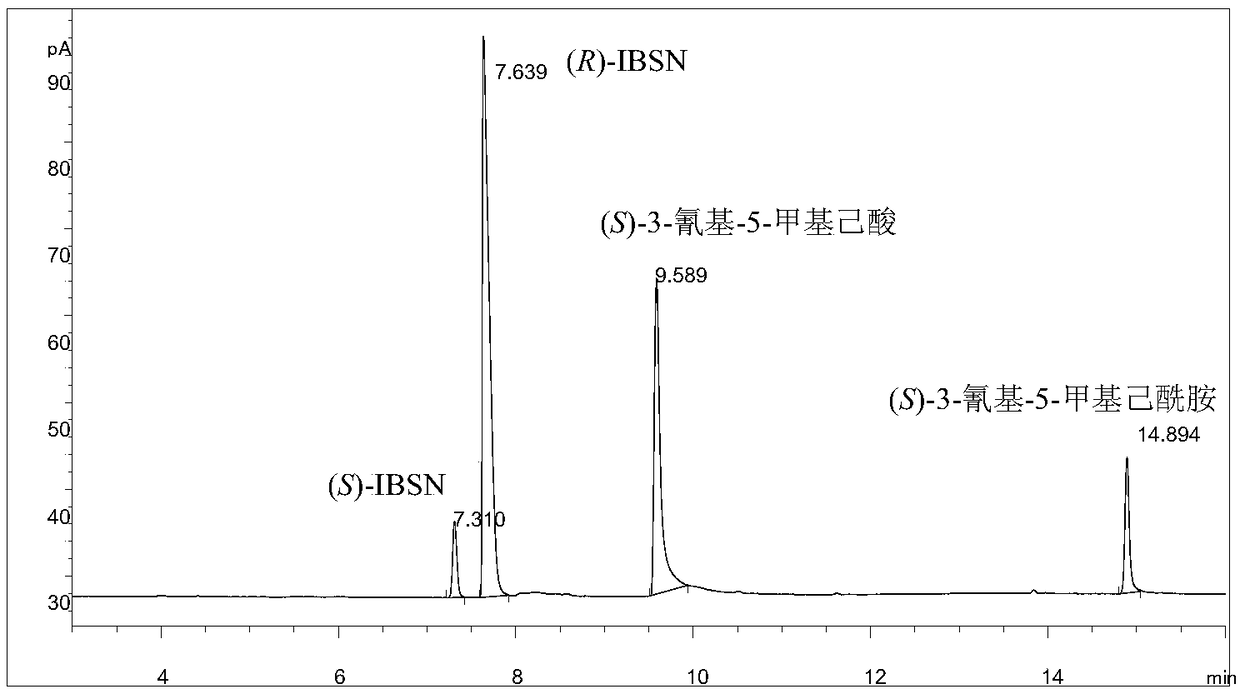
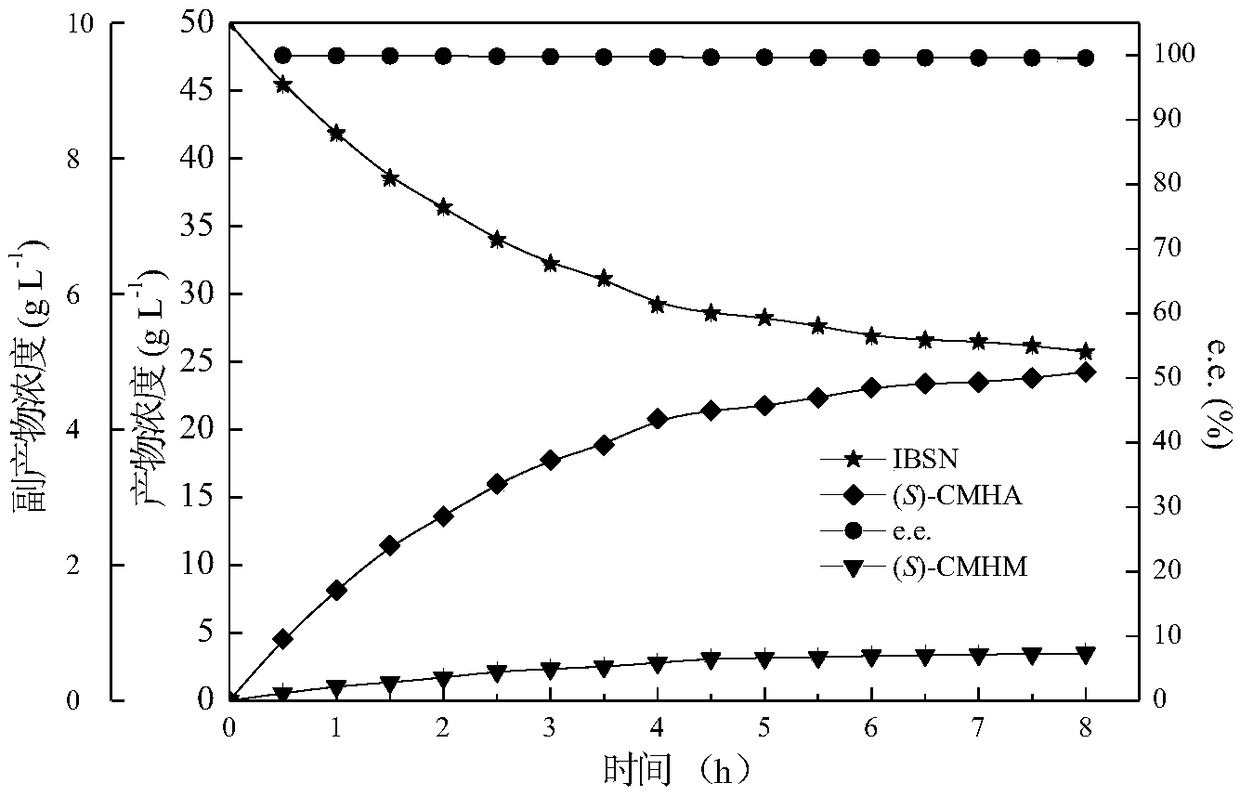
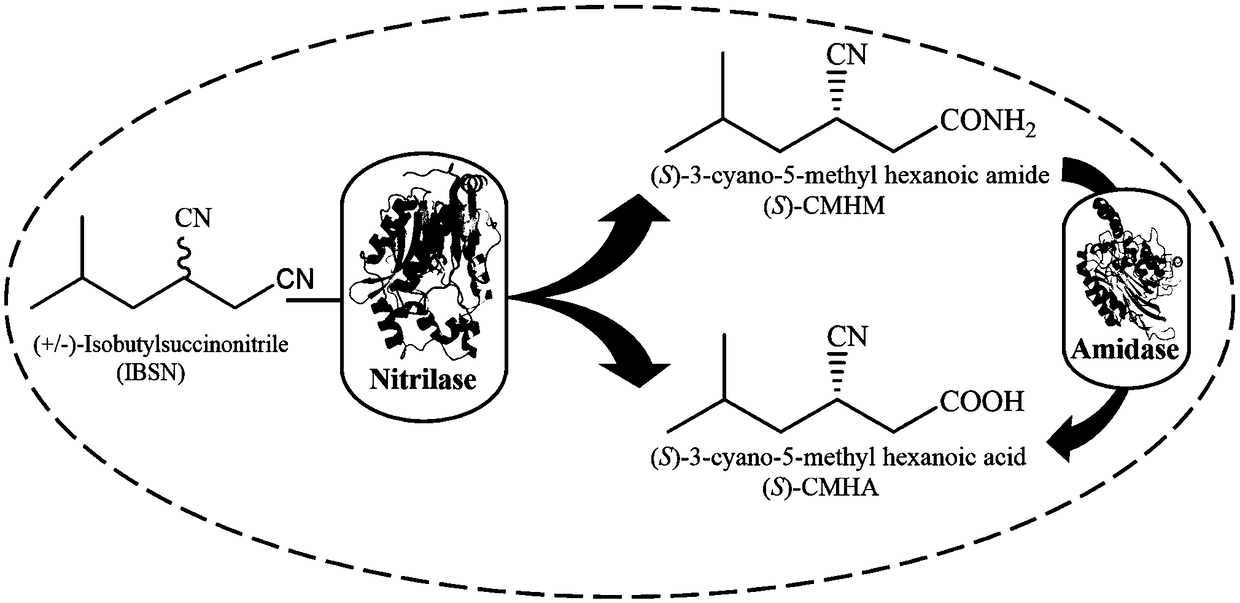
Method for synthesizing pregabalin chiral intermediate through regio-selective and stereo-selective bio-catalytic synthesis
A stereoselective, chiral intermediate technology, applied in fermentation and other directions, can solve the problems of many by-products, cumbersome product purification process, difficult to obtain both yield and optical purity, etc., and achieves simple operation, improved yield and optical purity. Effects with high purity, regio- and stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1 constructs recombinant nitrilase engineering bacterium
[0045] According to the report in NCBI, the turnip (Brassica rapa) nitrilase BrNIT (ABM55734.1), BrNIT2 (BAG72074), Arabidopsis thaliana (Arabidopsis thaliana) nitrilase AtNIT (NP 851011) and alpine Arabidopsis (Arabis alpine) nitrile The gene sequence was fully synthesized by the hydrolase AaNIT (KFK44999), and restriction sites XhoI and XbaI were designed at both ends, and cloned into the empty vector pET28b to obtain a recombinant plasmid, which was recovered and transformed into Escherichia coli BL21 (DE3), and the positive bacteria were cultured to obtain Recombinant nitrilase gene engineering bacteria BL21(DE3) / pET28b-BrNIT, BL21(DE3) / pET28b-BrNIT2, BL21(DE3) / pET28b-AtNIT and BL21(DE3) / pET28b-AaNIT.
Embodiment 2
[0046] Embodiment 2 constructs recombinant amidase engineering bacterium
[0047] According to the reported amidase genes Pa-Ami (WP_008109374), Cc-Ami (WP_027015397) and Dt-Ami (KP943494) derived from Pantoea sp.YR343, Comamonas composti and D.tsuruhatensis ZJB-05174, their gene sequences were total synthesis. Design upstream and downstream primers containing EcoRI and NcoI sites, insert the gene into pET28b after amplifying the gene, construct the expression vector, transform into BL21(DE3) competent cells by heat shock, and obtain amidase genetically engineered bacteria BL21(DE3) / pET28b- Pa-Ami, BL21(DE3) / pET28b-Cc-Ami and BL21(DE3) / pET28b-Dt-Ami.
Embodiment 3
[0048] The expression culture of embodiment 3 genetically engineered bacteria
[0049] Pick a single colony and inoculate it into 5 mL of liquid LB medium, and the final concentration of kanamycin is 50 mg / L. The culture conditions are 37° C., 200 rpm, and cultured for 6-8 hours. The above seed solution was transferred to fresh LB liquid medium containing 50mg / L kanamycin at a volume ratio of 2%, and cultivated at 37°C and 150rpm until the cell OD 600 At about 0.6-0.8, add isopropyl-β-D-thiogalactopyranoside (IPTG, final concentration: 0.1mM) to the above LB liquid medium, induce culture at 28°C, 150rpm for 10-12h, The fermented broth was collected and centrifuged at 4°C and 8000rpm for 10 minutes, then the cells were washed once with physiological saline, and the centrifuged cells were stored in a -20°C refrigerator for use in the following examples.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com