URAT1 inhibitor, preparation method and application thereof
The technology of a condensing agent and C1-C4 is applied in the field of medicines related to the treatment of hyperuricemia and gout, and can solve the problems of easily causing liver damage, large side effects, not many, etc. Excellent effect of lowering serum uric acid concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
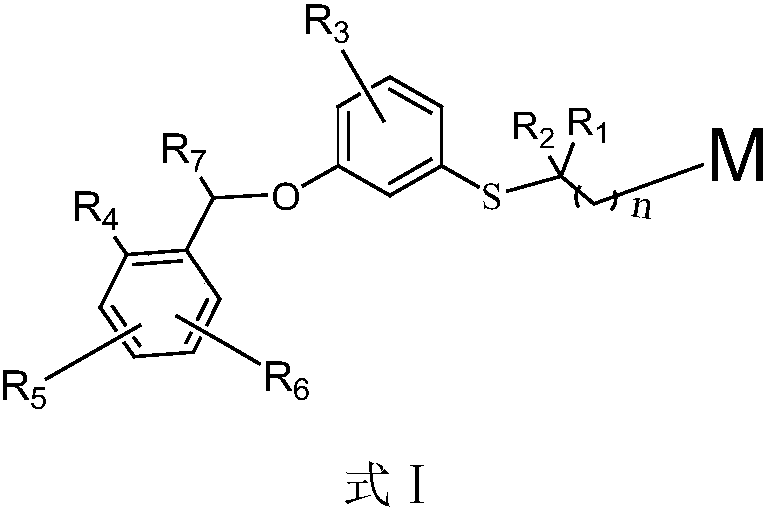
[0048] When M is a carboxylic acid, the compound of the formula I is a compound of the formula I', wherein the preparation method of the compound of the formula I' comprises:
[0049] The compound of formula II and the compound of formula III are heated and reacted under the action of a base to obtain the compound of formula IV, wherein the base is an organic base or an inorganic base, and the organic base is preferably selected from pyridine, 4-dimethylaminopyridine, triethylamine, Tributylamine or 1,8-diazabicycloundec-7-ene, the inorganic base is preferably selected from sodium hydride, lithium hydride, potassium carbonate, lithium carbonate, sodium carbonate, sodium bicarbonate or potassium bicarbonate;
[0050] The compound of formula IV is condensed with the compound of formula V under the action of a base or a condensing agent to obtain a compound of formula VI, wherein, when X in the compound of formula V is a halogen, OMs, OTs or OTf leaving group, the compound of form...
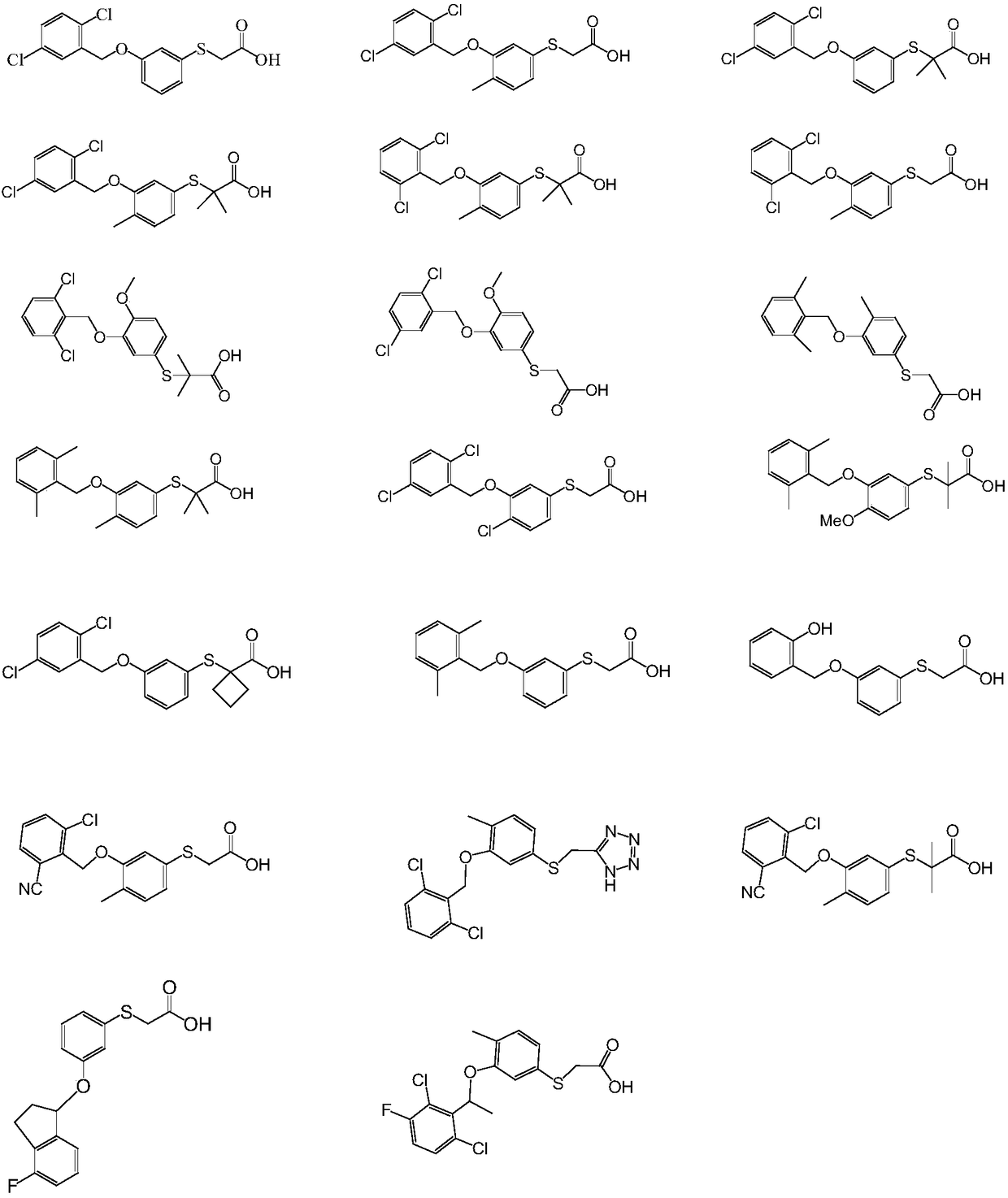
Embodiment 1
[0060] The synthesis of embodiment 1 compound 1
[0061]
[0062] Synthesis of Intermediate Compound A
[0063] 3-Mercaptophenol (12.6g, 100mmol), ethyl bromoacetate (16.7g, 100mmol) were added to 100mL of anhydrous DMF, and NaHCO 3 (8.4g, 100mmol), and heated to 60°C for 4 hours. Cooled to room temperature and poured into ice water, extracted with ethyl acetate, dried over anhydrous magnesium sulfate, filtered, concentrated under reduced pressure, separated by silica gel column chromatography (eluent: petroleum ether: ethyl acetate = 3:1) to obtain compound A , (18 g, 85% yield).
[0064] Synthesis of Intermediate Compound B
[0065] Compound A (4.2 g, 20 mmol) was dissolved in 30 mL of anhydrous DMF, and cooled to 0° C. in an ice-water bath. Add sodium hydrogen (800mg, 60% content, 20mmol) under stirring and keep stirring at 0°C for 15min, add 2,5-dichlorobenzyl bromide (5.3g, 22mmol), and naturally warm to room temperature after the addition, and stir overnight. Th...
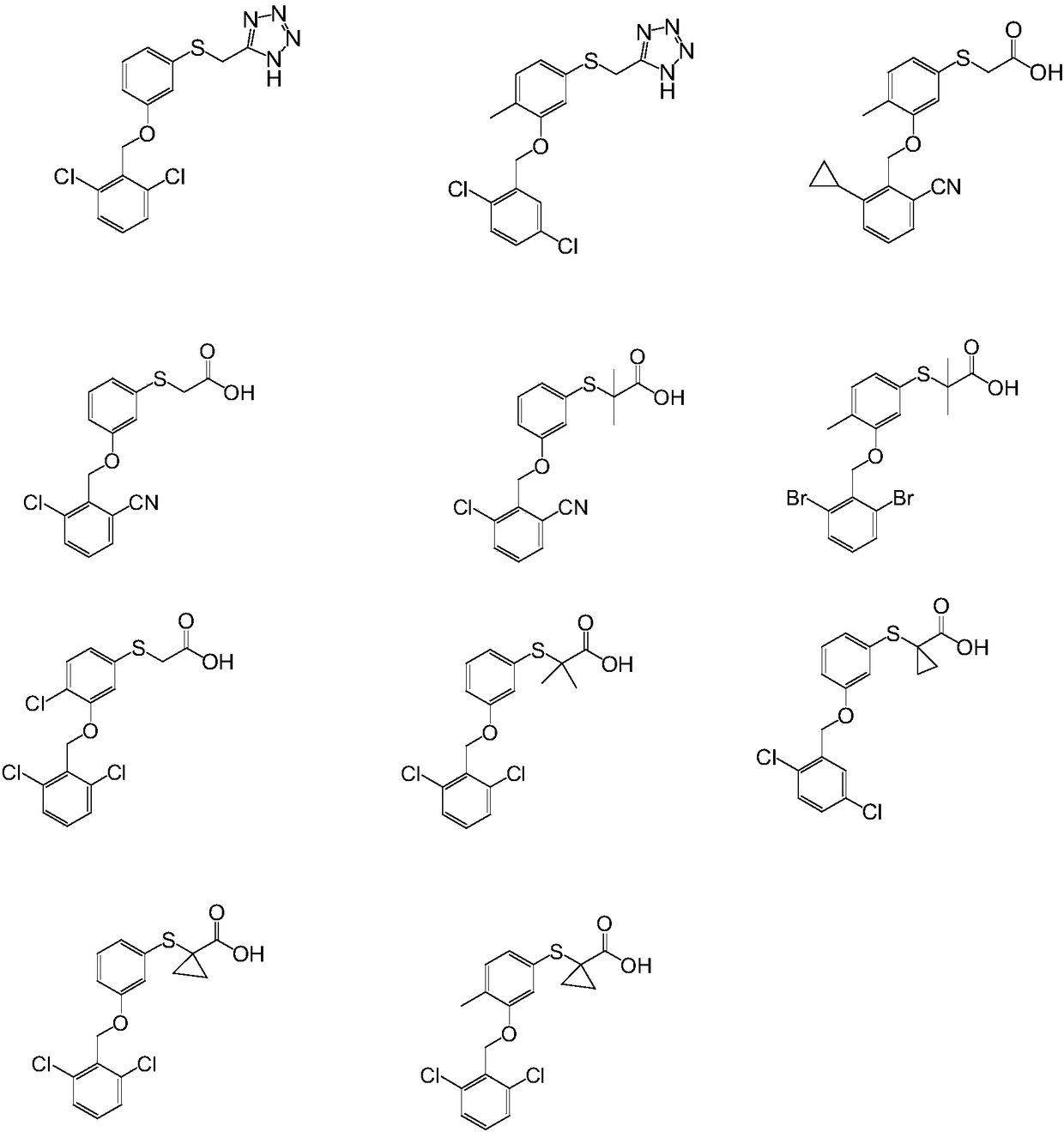
Embodiment 2-16
[0069] Select suitable raw materials and reagents, prepare compound 2-16 according to the method of Example 1:
[0070] Compound 2:
[0071]
[0072] MS m / z(ESI):357[M+1]; HNMR(400MHz d-DMSO) 12.74(s,1H),7.67(d,J=2.4Hz1H),7.57(d,J=8.4Hz,1H) ,7.47(dd,J=8.4Hz,J=2.8Hz 1H),7.12(d,J=7.6Hz,1H),7.07(s,1H),6.90(d,J=7.6Hz,1H),5.14( s,2H),3.78(s,2H),2.15(s,3H).
[0073] Compound 3:
[0074]
[0075] MS m / z (ESI): 393[M+Na]; HNMR (400MHz d-DMSO) 12.74(s, 1H), 7.68(d, J=2.4Hz1H), 7.56(d, J=8.4Hz 1H), 7.48(dd, J=2.4Hz, J=8.8Hz, 1H), 7.32(t, J=8.Hz, 1H), 7.06-7.12(m, 3H), 5.15(s, 2H), 1.38(s, 6H).
[0076] Compound 4:
[0077]
[0078] MS m / z (ESI): 385[M+1]; HNMR (400MHz CDCl 3 )7.54(d, J=2.0Hz 1H), 7.30(d, J=8.4Hz 1H), 7.23(dd, J=8.4Hz, J=2.0Hz, 1H), 7.12(d, J=7.6Hz, 1H ),7.05(d,J=8Hz,1H),7.01(s,1H),5.05(s,2H),2.3(s,3H),1.44(s,6H).
[0079] Compound 5:
[0080]
[0081]MS m / z(ESI):385[M+1]; HNMR(400MHz d-DMSO) 12.65(s,1H),7.58(d,J=8.4Hz2H),7.47(dd,J=8.8Hz,J= 7.2Hz 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com