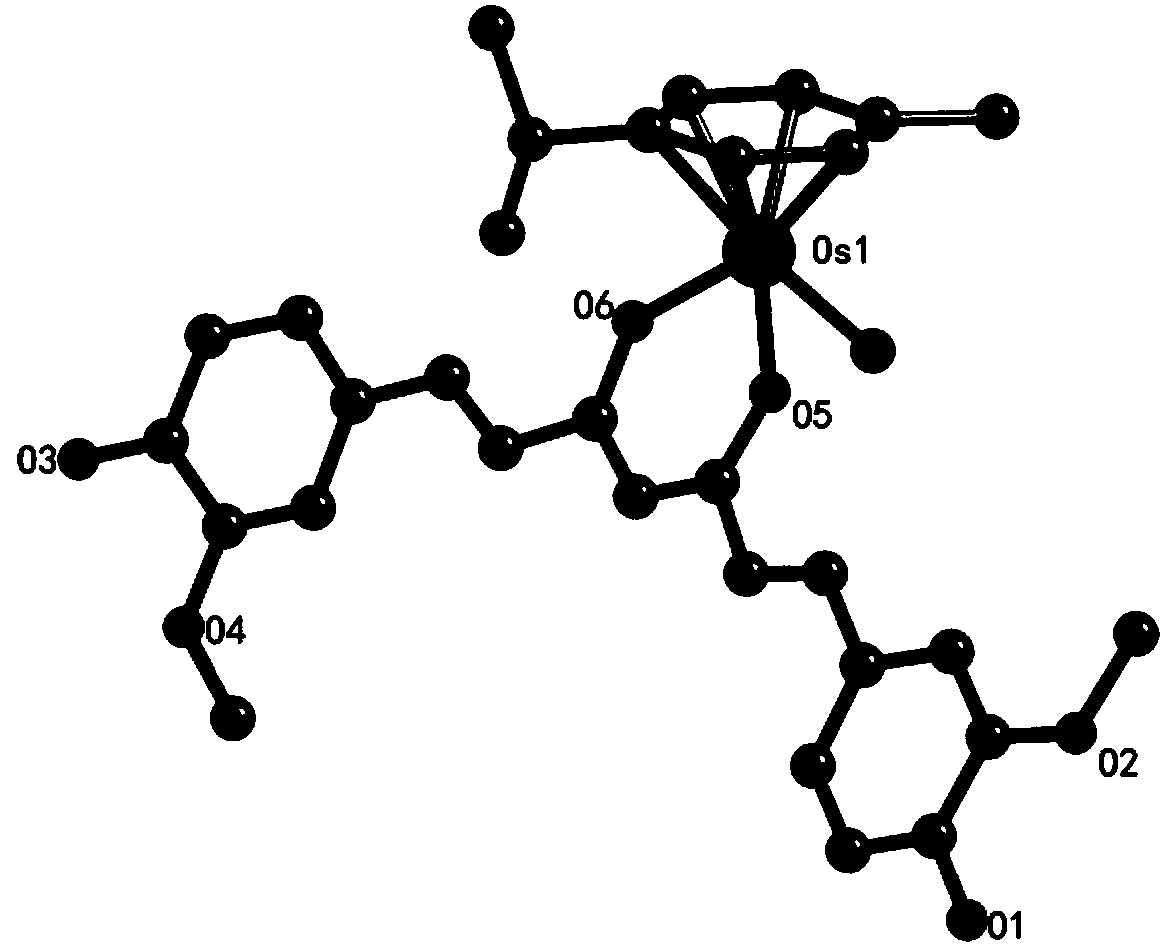
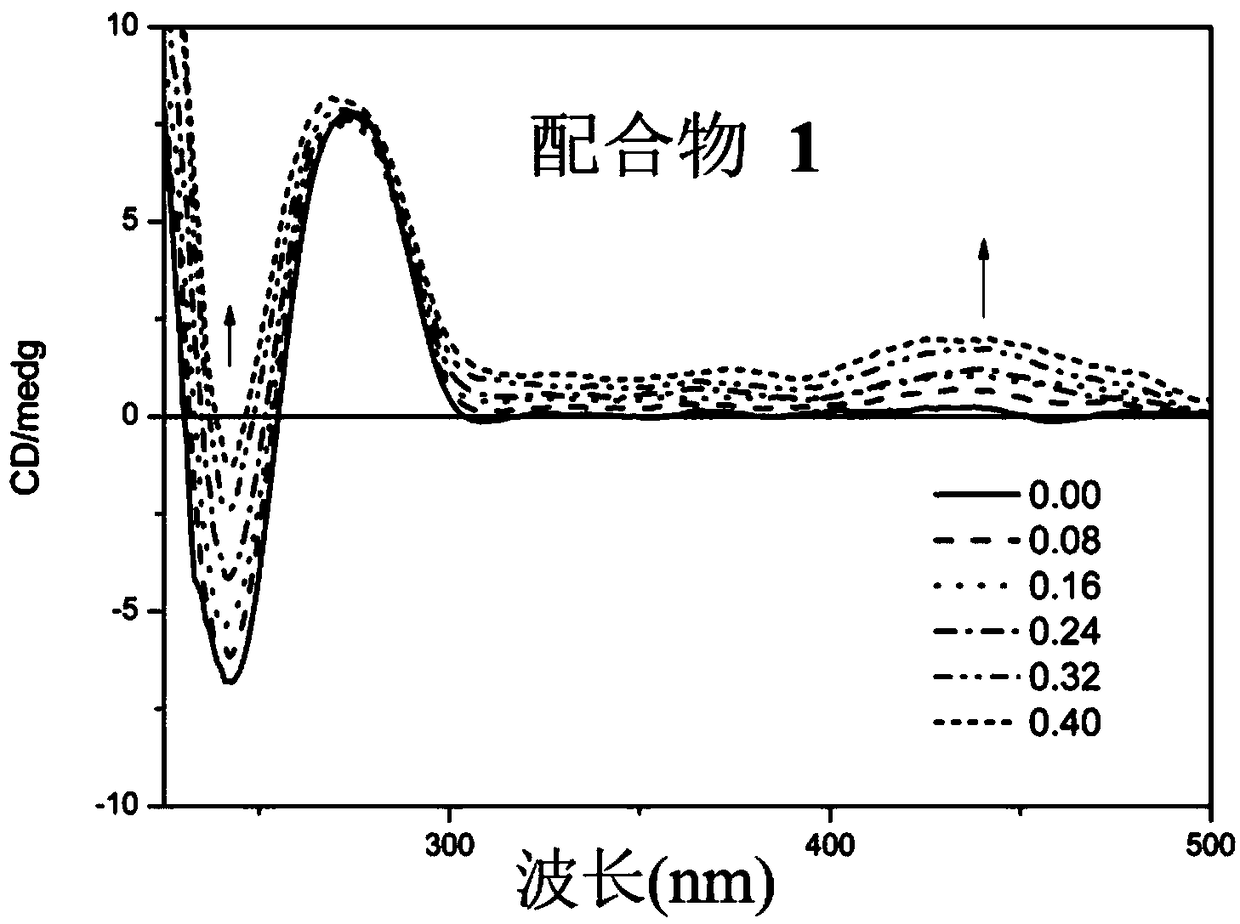
Curcumin-containing aryl metal complexes as well as synthetic method and application thereof
A technology of aryl metals and synthesis methods, applied in the direction of metallocenes, organic chemical methods, osmium organic compounds, etc., can solve the problems of high toxicity and side effects, difficult metabolism, low bioavailability, etc., to improve bioavailability and process flow Simple, cell death-inducing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Curcumin (18.40mg, 0.05mmol) and sodium methoxide (10.8mg, 0.20mmol) were dissolved in 20mL of methanol, stirred and reacted at room temperature for 1 hour; then dichloro(4-methylisopropylphenyl) Osmium(II) dimer (39.54mg, 0.05mmol) was added to the reaction tube (the mixed reaction material was reacted in the reaction tube), stirred and reacted at 48°C under reflux for 24h; In addition to the organic solvent, select a suitable eluent to separate and purify by column chromatography, place the separated and purified product for recrystallization in a refrigerator, and slowly precipitate to obtain orange-red crystals, collect the crystals, vacuum dry, weigh, Calculated yield: 68%.
[0040] Elemental analysis: Theoretical value (%): C 31 h 33 o 6 ClOs (CH 3 OH)·0.5(H 2 O): C50.02, H4.99; Experimental value: C49.84, H4.88. 1 H NMR (400MHz, DMSO) δ9.56(s, 2H), 7.41(d, J=15.6Hz, 2H), 7.24(d, J=1.7Hz, 2H), 7.06(dd, J=8.2, 1.7Hz , 2H), 6.81(d, J=8.1Hz, 2H), 6.56(d, J=15....
Embodiment 2
[0042] Curcumin (18.40mg, 0.05mmol) and potassium hydroxide (2.8mg, 0.05mmol) were dissolved in 15mL of ethylene glycol, stirred and reacted at room temperature for 0.5 hours; then dichloro(biphenyl)osmium(II ) dimer (33.23mg, 0.04mmol) was added to the reaction tube (the mixed reaction material was reacted in the reaction tube), stirred and reacted at 86°C under reflux for 17h; after the reaction was complete, the organic solvent was removed under reduced pressure distillation , select a suitable eluent to separate and purify by column chromatography, place the separated and purified product for recrystallization in a refrigerator, and slowly precipitate to obtain orange-red crystals, collect the crystals, vacuum dry, weigh, and calculate the yield For: 73%.
[0043] Elemental analysis: theoretical value (%) C 33 h 29 o 6 ClOs 0.5(CH 3 OH): C53.04, H3.91; Experimental value: C52.24, H3.87. 1H NMR (400MHz, DMSO) δ9.54(s, 2H, OH of curc), 7.78(dd, J=6.5, 3.1Hz, 2H, C(4,4')...
Embodiment 3
[0045] Curcumin (92.0mg, 0.25mmol) and sodium hydroxide (2.0mg, 0.05mmol) were dissolved in 20mL of dichloromethane, stirred and reacted at room temperature for 2 hours; then dichloro(biphenyl)ruthenium(II ) dimer (9.79mg, 0.015mmol) was added to the reaction tube (the mixed reaction material was reacted in the reaction tube), stirred and reacted at 25°C under reflux for 44h; after the reaction was complete, the organic solvent was removed under reduced pressure distillation , select a suitable eluent to separate and purify by column chromatography, place the separated and purified product for recrystallization in a refrigerator, and slowly precipitate to obtain orange-red crystals, collect the crystals, vacuum dry, weigh, and calculate the yield For: 80%.
[0046] Elemental analysis: theoretical value (%) C 33 h 29 o 6 C1Ru·0.5(CH 3 OH): C60.23, H4.44; found: C59.01, H 4.17. 1 H NMR (500MHz, CDCl 3 )δ7.81(dd, J=6.6, 2.7Hz, 2H, C(4,4')H of curc), 7.50(m, 3H, H bip ), 7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com