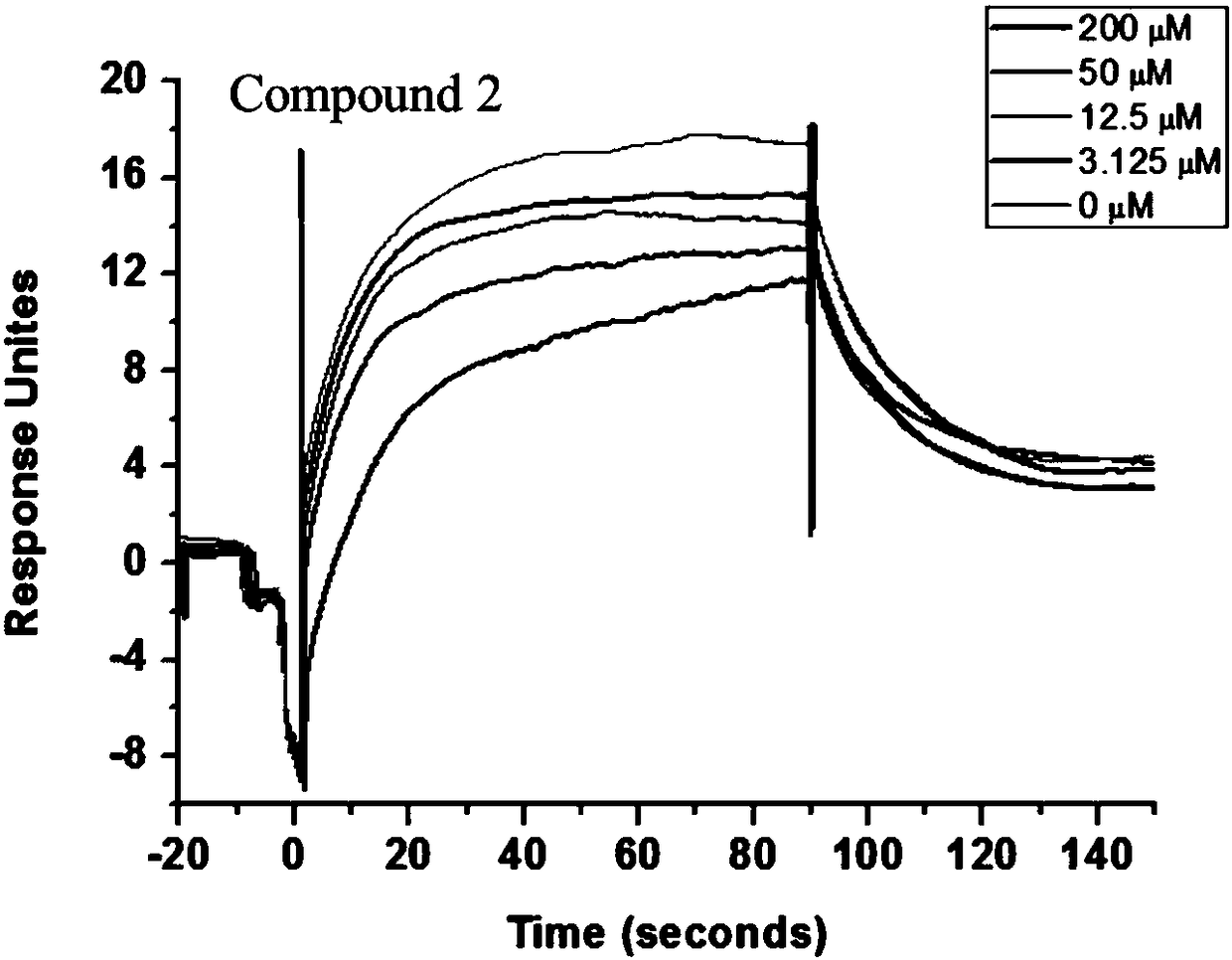
Novel PD-1 inhibitor and application thereof
A PD-1 and inhibitor technology, which is applied in the direction of anti-inflammatory agents, non-central analgesics, medical preparations containing active ingredients, etc., can solve the problems of easy immunogenicity and high production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0163] Example 1. Human PD-1 expression vector construction
[0164] According to relevant literature, the target gene was selected as the 34-150 amino acids of human PD-1. The target gene was cloned into the pET-28a vector using NCoI and NdeI restriction sites. Firstly, specific primers were designed according to the two restriction sites of NCoI and NdeI to amplify the human PD-1 gene by PCR. Then use the traditional cloning method to double digest the carrier plasmid and PCR product with two restriction enzymes NCoI and NdeI respectively, then connect them under the action of T4 ligase to form a recombinant plasmid, and finally transform the recombinant plasmid into the large intestine Bacillus DH5α competent cells. Single clones were picked for identification after overnight culture.
Embodiment 2
[0165] Example 2. Expression and purification of human PD-1 protein
[0166] Select the bacterial liquid with a completely matching sequence after the company's sequencing and culture it overnight, extract the plasmid, and then transform the plasmid into the expression host E.coli BL21(DE3) for expression. A single clone transformed into E.coli BL21(DE3) was picked, placed in 20 mL of 2×YT medium containing kanamycin, and cultured in a shaker at 37° C. overnight. The next day, the small culture products were transferred to TB medium containing kanamycin, cultured at 37°C until the OD600 was 0.6-0.8, and induced by adding 0.5mM IPTG at 37°C for 5-7h. Collect bacteria by centrifugation at 4000rpm, lyse Escherichia coli with lysis buffer (50mM Tris-HCl, pH 8.0, 50mM NaCl, 1mM DTT, 0.5mMEDTA, 5% glycerol), then high-pressure crush, centrifuge at 12000rpm for 60min, take precipitation. Wash with washing buffer (20 mM Tris-HCl, pH 8.0, 2M urea, 2.5% Triton X-100) three times, and ...
Embodiment 3
[0167] Example 3. Identification of human PD-1 protein
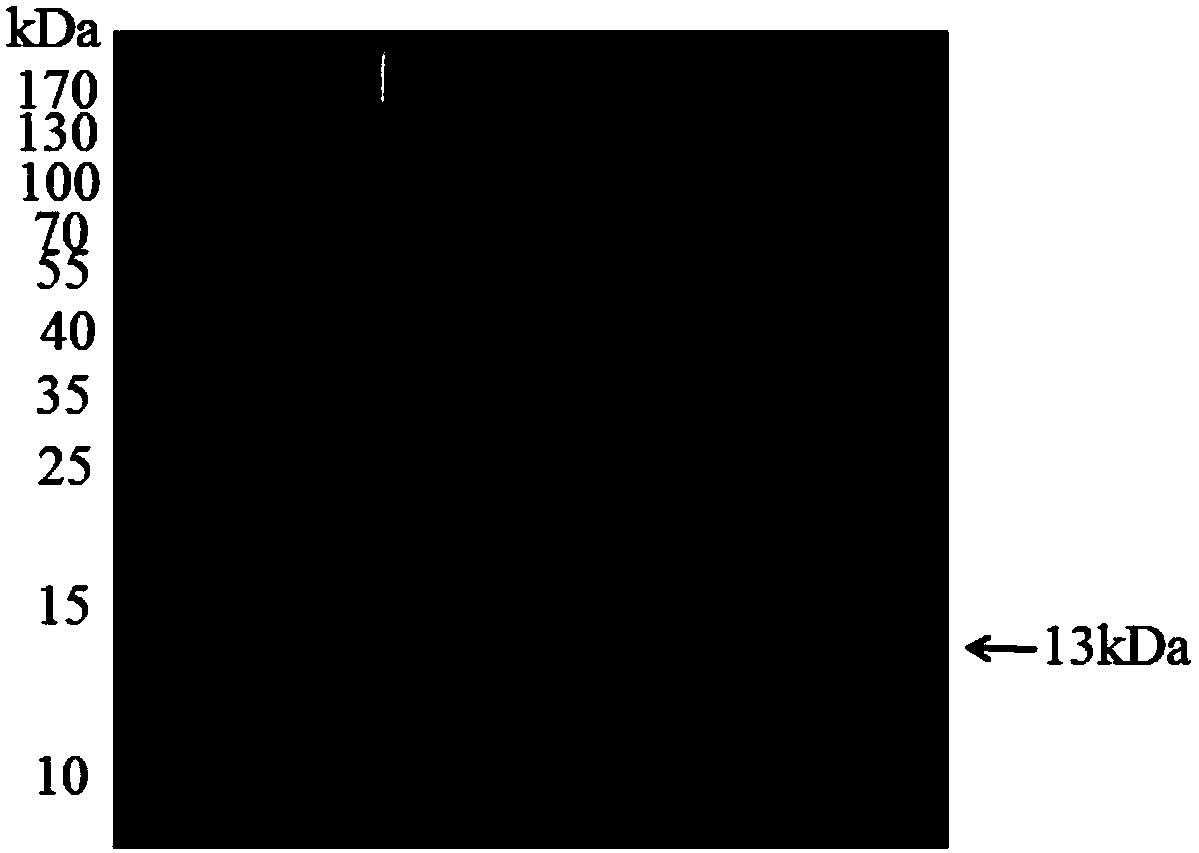
[0168] The purified human PD-1 protein was subjected to 14% SDS-PAGE gel electrophoresis, and then transferred to a membrane. The transfer current was set to 300mA, and the transfer time was 45min. The membrane was then blocked with 5% skim milk powder for 2 h at room temperature. After removal, incubate overnight at 4°C with mouse anti-human PD-1 monoclonal antibody, rinse three times in 1×TBST solution, incubate with goat anti-mouse monoclonal antibody at room temperature for 2 hours, and rinse three times in 1×TBST solution. After adding the developing solution, develop under the automatic chemiluminescence image analysis system. The results are shown in 2, there is a protein band between 10-15kDa, and it can be visualized under the PD-1 antibody, thus indicating that the purified protein is indeed human PD-1 protein.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com