The preparation method of calcium dihydrogen phosphate
A technology of calcium dihydrogen phosphate and phosphoric acid, applied in chemical instruments and methods, fluorosilicic acid, calcium fertilizers, etc., can solve the problems of low utilization efficiency of raffinate acid and great soil damage, and improve the dissolution rate of phosphate rock. , The effect of saving sulfuric acid and phosphorus yield improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
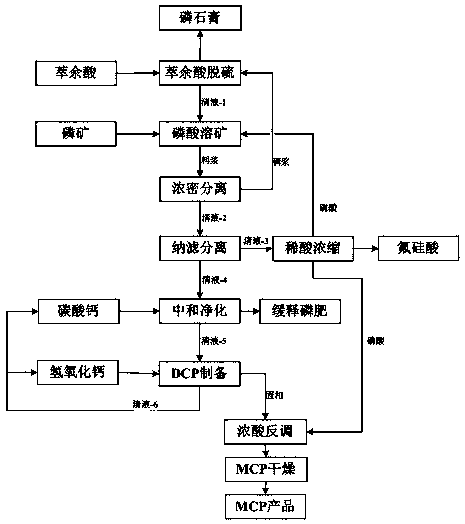
[0036] A preparation method of calcium dihydrogen phosphate, comprising:
[0037] Step 1: Use raffinate to react with the thick slurry returned in step 3, separate the phosphogypsum produced, and obtain clear liquid-1, the ratio of the dry weight of phosphogypsum to the phosphate rock consumed in step 2 is 0.5:1, and the P in phosphogypsum 2 o 5 Quality fraction 1.0%;
[0038] Step 2: Mix clear liquid-1 obtained in step 1, phosphate rock, and phosphoric acid obtained in step 9 in a mass ratio of 1:1:10, the reaction time is 30 minutes, and the reaction temperature is 90°C;
[0039] Step 3: Thicken the slurry in step 2 with a thickener to obtain the clear liquid-2 and the bottom dense slurry, and return the thick slurry to step 1 for use;
[0040] Step 4: The supernatant-2 is separated and purified through the nanofiltration membrane to obtain phosphoric acid separation and purification, and the supernatant-3 with a low mass fraction of cations is obtained and sent to step 9,...
Embodiment 2
[0049] A preparation method of calcium dihydrogen phosphate, comprising:
[0050] Step 1: Use raffinate to react with the thick slurry returned in step 3, separate the phosphogypsum produced, and obtain clear liquid-1. The ratio of the dry weight of phosphogypsum to the phosphate rock consumed in step 2 is 0.6:1, and the P in phosphogypsum 2 o 5 Mass fraction 0.75%;
[0051] Step 2: Mix clear liquid-1 obtained in step 1, phosphate rock, and phosphoric acid obtained in step 9 in a mass ratio of 10:1:5, the reaction time is 60 minutes, and the reaction temperature is 80°C;
[0052] Step 3: Thicken the slurry in step 2 with a thickener to obtain the clear liquid-2 and the bottom dense slurry, and return the thick slurry to step 1 for use;
[0053] Step 4: The supernatant-2 is separated and purified through the nanofiltration membrane to obtain phosphoric acid separation and purification, and the supernatant-3 with a low mass fraction of cations is obtained and sent to step 9, a...
Embodiment 3
[0062] A preparation method of calcium dihydrogen phosphate, comprising:
[0063] Step 1: Use raffinate to react with the thick slurry returned in step 3, separate the phosphogypsum produced, and obtain clear liquid-1, the ratio of the dry weight of phosphogypsum to the phosphate rock consumed in step 2 is 0.8:1, and the P in phosphogypsum 2 o 5 Quality fraction 0.65%;
[0064] Step 2: Mix clear liquid-1 obtained in step 1, phosphate rock, and phosphoric acid obtained in step 9 in a mass ratio of 15:1:3, the reaction time is 90 minutes, and the reaction temperature is 80°C;
[0065] Step 3: Thicken the slurry in step 2 with a thickener to obtain the clear liquid-2 and the bottom dense slurry, and return the thick slurry to step 1 for use;
[0066] Step 4: The supernatant-2 is separated and purified through the nanofiltration membrane to obtain phosphoric acid separation and purification, and the supernatant-3 with a low mass fraction of cations is obtained and sent to step 9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com