Enzyme-sensitive and redox-sensitive dual response type copolymer as well as preparation method and application thereof
A dual-response, enzyme-sensitive technology, applied in the fields of polymer chemistry and pharmaceutical preparations, can solve problems such as lack of pertinence, worsening side effects, and limited effects, and achieve good biocompatibility, reduce toxic side effects, and improve environmental sensitivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
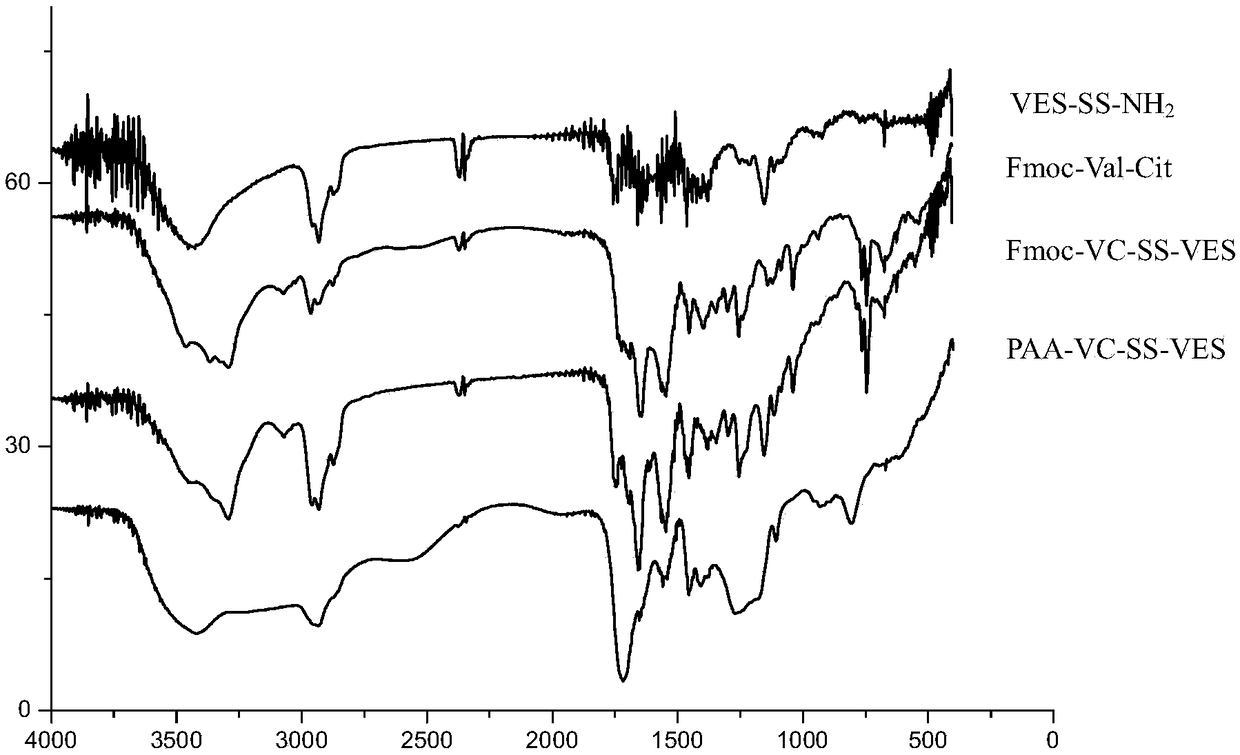
[0034] Example 1 Polyacrylic acid-VC-SS-vitamin E succinate copolymer PAA-VC-SS-VES
[0035]
[0036] (1) The preparation method is as follows:
[0037] 1. Vitamin E succinate derivative VES-SS-NH 2 (Intermediate A) synthetic, concrete steps are as follows:
[0038]
[0039] Dissolve vitamin E succinate (VES) (5.30g, 10mmol) in 100mL of dichloromethane, stir in a 250mL round-bottomed flask until completely dissolved, add EDC (13mmol) and HOBT (13mmol) in an ice bath, and store in room temperature. Stir overnight, add cystamine dihydrochloride (6.75g, 30mmol) to the reaction solution, add 30mL of methanol to aid dissolution, add triethylamine to adjust the pH to 7-8, stir for 24h, the obtained product is treated with 1mol / L NaHCO 3 Wash with aqueous solution, dry the organic layer by adding anhydrous magnesium sulfate, filter, remove dichloromethane by rotary evaporation under reduced pressure at 40°C, and dry in vacuum to obtain 4.72g vitamin E succinate derivative VE...
Embodiment 2
[0051] Embodiment 2 PAA-VC-SS-VES micelles and drug-loaded nano micelles
[0052] (1) Determination of appearance, particle size and potential of PAA-VC-SS-VES micelles
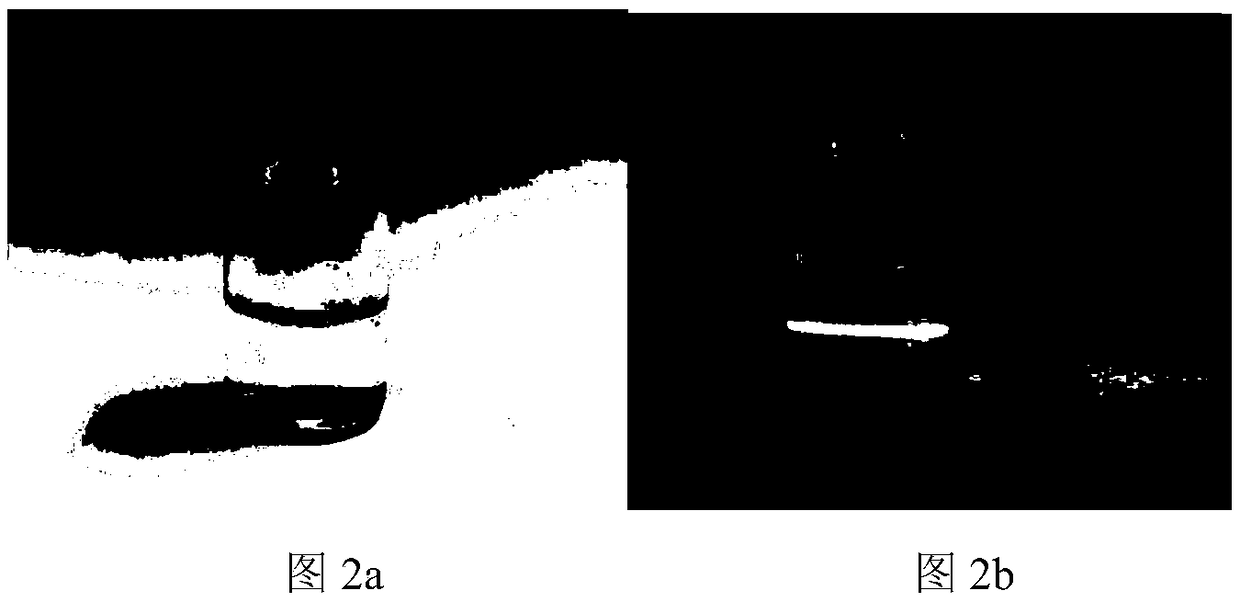
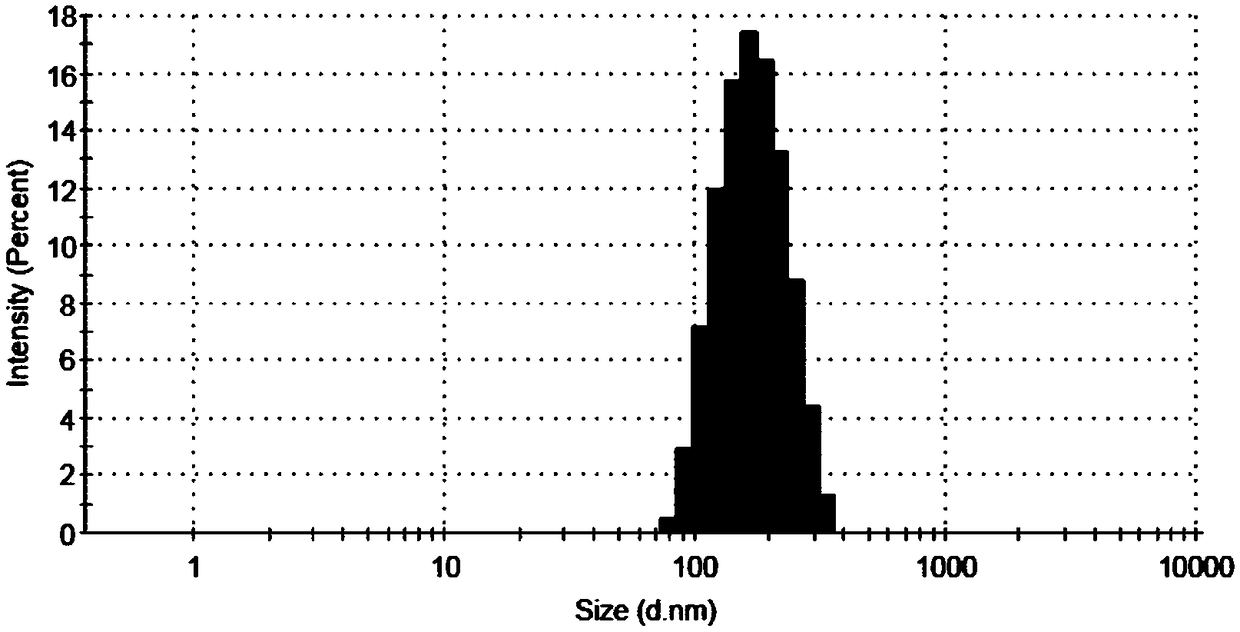
[0053] Get 10mg PAA-VC-SS-VES copolymer and dissolve in 10mL distilled water, stir at room temperature for ten minutes, the appearance of the preparation is clear and transparent, as Figure 2a It shows that the solubility of the copolymer is excellent. Irradiating the preparation with a beam of light showed a bright path, indicating that the PAA-VC-SS-VES copolymer can spontaneously form nanomicelles in water (such as Figure 2b shown). Detect with the Malvern laser particle size scanner, record that this nano-micelle particle size is 147.7nm (as Figure 2c shown), the potential is -23.2mV (such as Figure 2d shown), indicating that the nano-micelle has a small particle size, can be enriched at the tumor site, and has good stability and dispersion.
[0054] (2) Preparation of PAA-VC-SS-VES drug-loaded n...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com