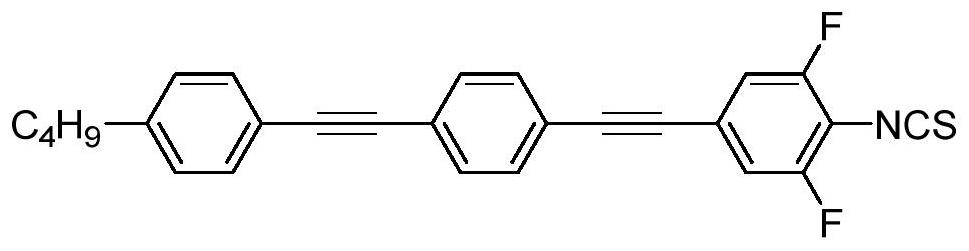
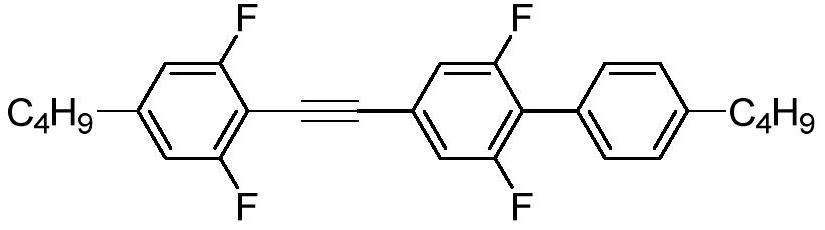
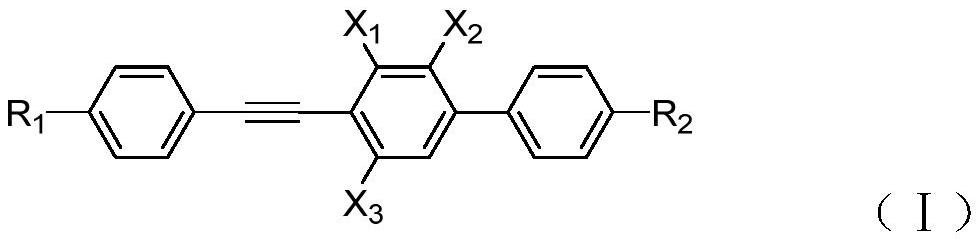
A liquid crystal compound, a preparation method, a composition containing the compound, and a high-frequency component containing the liquid crystal medium
A technology for liquid crystal compositions and compounds, applied in chemical instruments and methods, liquid crystal materials, etc., can solve problems such as reduction, increase in dielectric tuning rate and dielectric loss, and achieve simple post-processing process, high quality factor, easy effect of operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Synthesis of 2-chloro-4'-ethyl-4-((4-propylphenyl)ethynyl)-1,1'-biphenyl:
[0037] The specific structure is as follows:
[0038]
[0039] The preparation process is as follows:
[0040] Step 1: Synthesis of 1-bromo-2-chloro-4-((4-propylphenyl)ethynyl)benzene
[0041]
[0042] Under nitrogen protection, 1-bromo-2-chloro-4-iodobenzene (10 g, 0.0315 mol), bis(triphenylphosphine) palladium dichloride (0.22 g, 1% mol), iodine Cuprous chloride (0.18g, 3%mol), PPh 3 (0.25g, 3%mol), triethylamine 50mL. Propylphenylacetylene (4.54 g, 0.0315 mol) was added dropwise at 10° C. After the drop, the mixture was reacted for 2 h under heat preservation for post-treatment. Filter the reaction solution, add n-heptane to dissolve the filtrate after rotary evaporation, wash with saturated ammonium chloride aqueous solution twice until neutral, dry over anhydrous magnesium sulfate, filter, and perform column chromatography on the filtrate, elute with n-heptane, elute After liquid r...
Embodiment 2
[0055] Synthesis of 2-chloro-4'-propyl-4-((4-propylphenyl)ethynyl)-1,1'-biphenyl
[0056] The specific structure is as follows:
[0057]
[0058] Propylphenylboronic acid is used to replace the ethylphenylboronic acid in the step (2) of Example 1, and the same method as in Example 1 is used to synthesize 2-chloro-4'-propyl-4-((4-propylphenyl )ethynyl)-1,1'-biphenyl.
[0059] Structure Identification: 1 H NMR (δ, CDCl 3 ):0.964-0.993(t,3H),1.003-1.032(t,3H),1.647-1.750(m,4H),2.623-2.690(m,4H),7.197-7.213(d,J=8Hz,2H) ,7.274-7.287(m,2H),7.335-7.351(d,J=8Hz,1H),7.398-7.414(m,2H),7.460-7.492(m,3H),7.657-7.660(d,1H); MS(70eV)m / z(%):372.2(M + ,100),343.1(74),314.1(26),276.1(8),157.1(19),139.1(7).
[0060] The above structural identification data show that the synthesized compound is indeed 2-chloro-4'-propyl-4-((4-propylphenyl)ethynyl)-1,1'-biphenyl.
[0061] Use DSC to test the liquid crystal phase transition temperature of 2-chloro-4'-propyl-4-((4-propylphenyl)ethynyl)-1,...
Embodiment 3
[0063] Synthesis of 4'-butyl-2-chloro-4-((4-propylphenyl)ethynyl)-1,1'-biphenyl
[0064] The specific structure is as follows:
[0065]
[0066] Using butylphenylboronic acid instead of ethylphenylboronic acid in step (2) of Example 1, the same method as in Example 1 was used to synthesize 4'-butyl-2-chloro-4-((4-propylphenyl )ethynyl)-1,1'-biphenyl.
[0067] Structure Identification: 1 H NMR (δ, CDCl 3 ):0.967-1.004(m,6H),1.399-1.473(m,2H),1.650-1.725(m,4H),2.626-2.656(t,2H),2.685-2.716(t,2H),7.200-7.216 (d, J=8Hz, 2H), 7.278-7.294(m, 2H), 7.335-7.351(d, J=8Hz, 1H), 7.398-7.414(m, 2H), 7.461-7.496(m, 3H), 7.660-7.663(d,1H); MS(70eV)m / z(%):386.2(M + ,100),343.1(38),314.0(25),276.1(6),157.1(11),139.1(6).
[0068] The above structural identification data show that the synthesized compound is indeed 4'-butyl-2-chloro-4-((4-propylphenyl)ethynyl)-1,1'-biphenyl.
[0069] Use DSC to test the liquid crystal phase transition temperature of 4'-butyl-2-chloro-4-((4-propylphenyl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com