Recombination chimeric antigen receptor gene and application thereof
A technology of recombining genes and antigens, which is applied in the field of tumor treatment, can solve the problems of low specificity, insufficient proliferation ability of T cells, and insufficient effect, and achieve the effect of high transfection rate, high specificity, and good proliferation ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
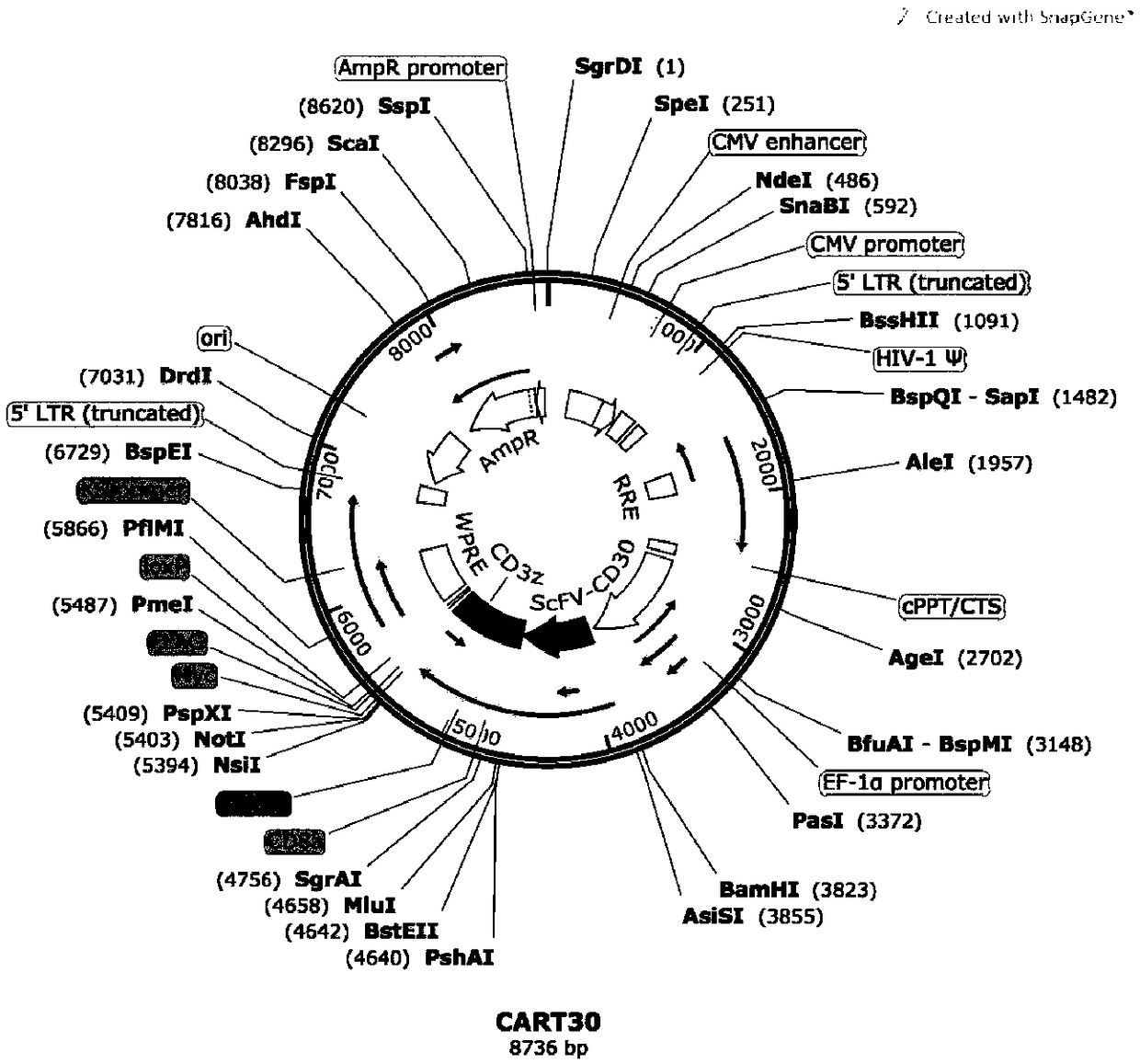
[0088] Embodiment 1: Construction of CART-30scFv plasmid
[0089] The method for obtaining the CART-30 scFv plasmid: design the coding nucleotide sequences of the scFv of 3 CD30 antibodies, including the CD8a signal peptide coding sequence whose sequence is SEQ ID NO: 26 and shown by SEQ ID NO: 23, 24 and 25 respectively The coding sequence of the scFv fragment is connected with the sequence shown in SEQ ID NO: 11, 12 and 13 to form 3 CAR genes:
[0090] CAR1: consists of SEQ ID NO: 26, 23, 11, 12 and 13;
[0091] CAR2: consists of SEQ ID NO: 26, 24, 11, 12 and 13;
[0092] CAR3: consists of SEQ ID NO: 26, 25, 11, 12 and 13;
[0093] The sequence of CAR3 is SEQ ID NO: 21;
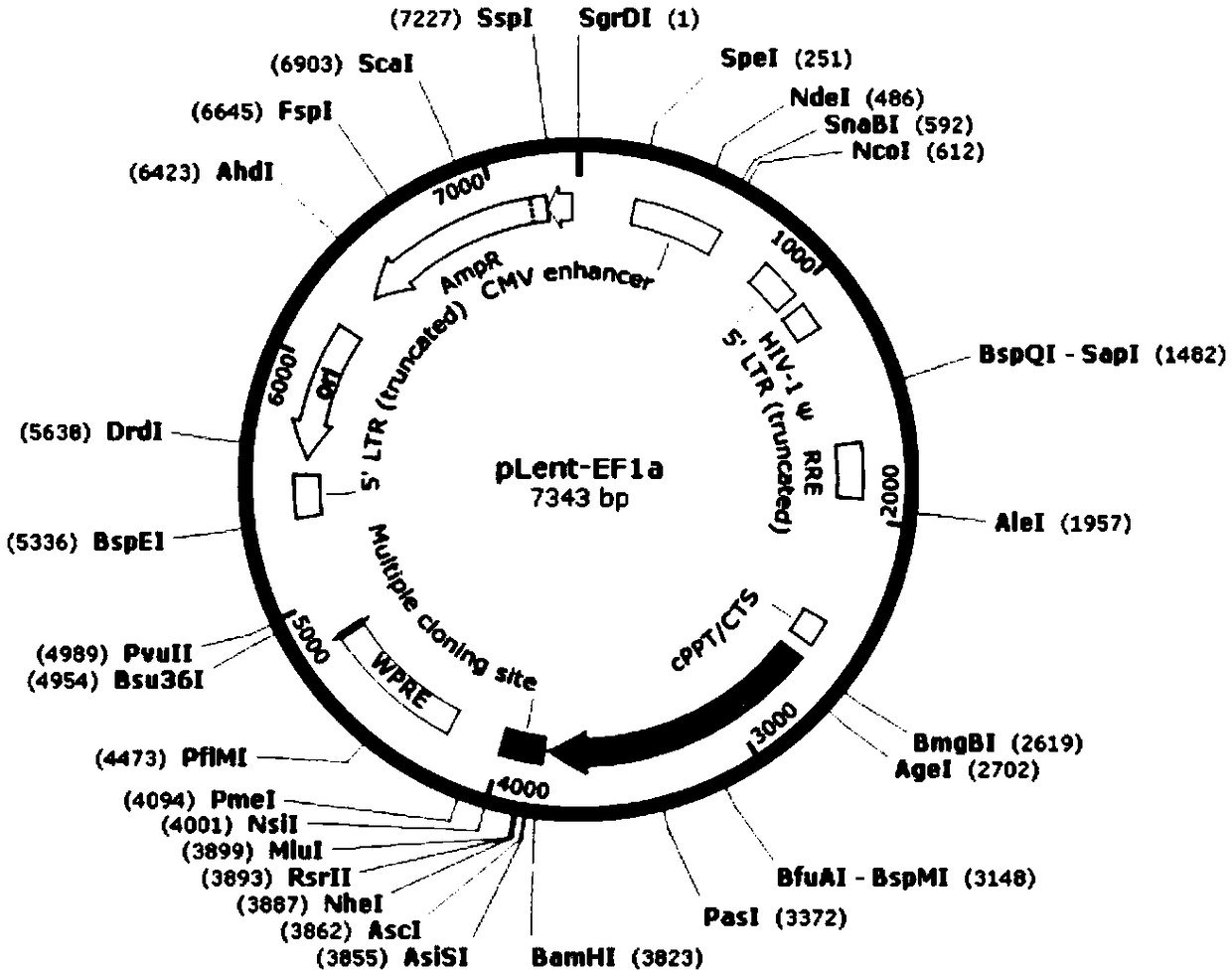
[0094] The complete sequences of the recombinant genes CAR1, CAR2 and CAR3 were synthesized (completed by GenScript Biotechnology Co., Ltd.), and respectively ligated into the lentiviral plasmid vector pLent-EF1a (provided by Weizhen Biotechnology Co., Ltd.) by AsisI / NsiI (NEB Company). Technology Co., ...
Embodiment 2
[0095] Example 2: T lymphocyte transfection
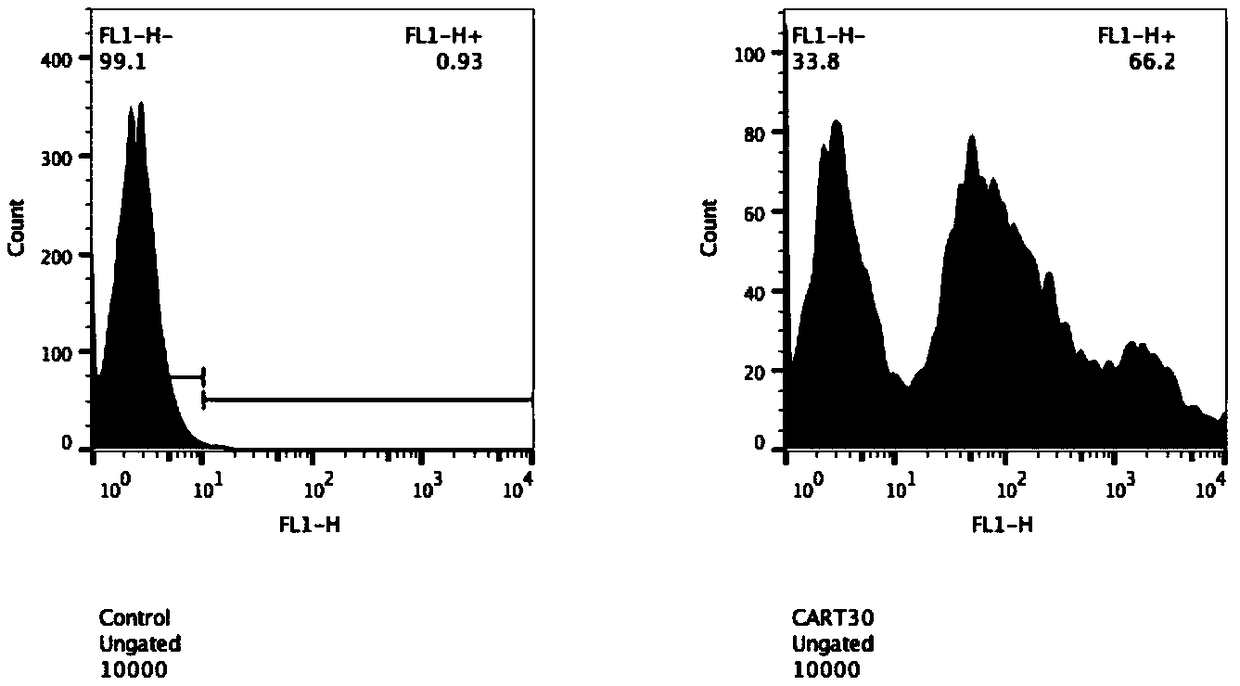
[0096] The CART-30scFv plasmid constructed in Example 1 was packaged and purified by lentivirus, and then using lentiviral vector technology (references: Tumaini B, Lee DW, Lin T, Castiello L, et al. Simplified process for the production of anti-CD19 -CAR engineered T cells.Cytotherapy.2013; 15(11):1406-1415) expressed three recombinant CAR genes in T lymphocytes, and used flow cytometry to detect the positive rate of infection. The results are shown in image 3 and 4 , the transfection efficiency of lentivirus-infected T lymphocytes is about 60%.
Embodiment 3
[0097] Embodiment 3: Detection of killing rate in vitro
[0098] 1. Construction of CAR-T cytotoxicity indicator vector
[0099] According to the method described in the applicant's previous Chinese patent 201610537806.3, it specifically includes the following steps:
[0100] (1) Composite code The nucleotide sequences of Luciferase, P2A and CD30 are SEQ ID NO: 30, and Asis I and MluI enzyme cutting sites are respectively introduced upstream and downstream;
[0101] (2) Use Asis I and MluI to digest Plent-EF1α-Puro-CMV vector and step (1) at 37°C Luciferase-P2A-CD30 sequence, use DNA gel extraction kit to recover enzyme-digested Luciferase-P2A-CD30 sequence and Plent-EF1α-Puro-CMV vector;
[0102] (3) Digested The Luciferase-P2A-CD30 sequence and the Plent-EF1α-Puro-CMV vector were ligated at 22°C for 2 hours, and the ligation product was transformed into Escherichia coli DH5α competent cells by chemical method to obtain the cytotoxicity indicator vector pLent-EF1a-Nl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com