Tetrazine ring polycarbazole-based nitrogen-doped carbon-oxygen reduction catalyst and preparation method and application thereof
A nitrogen-doped carbon and catalyst technology, applied in electrical components, battery electrodes, circuits, etc., can solve problems such as low specific surface area and low ORR catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
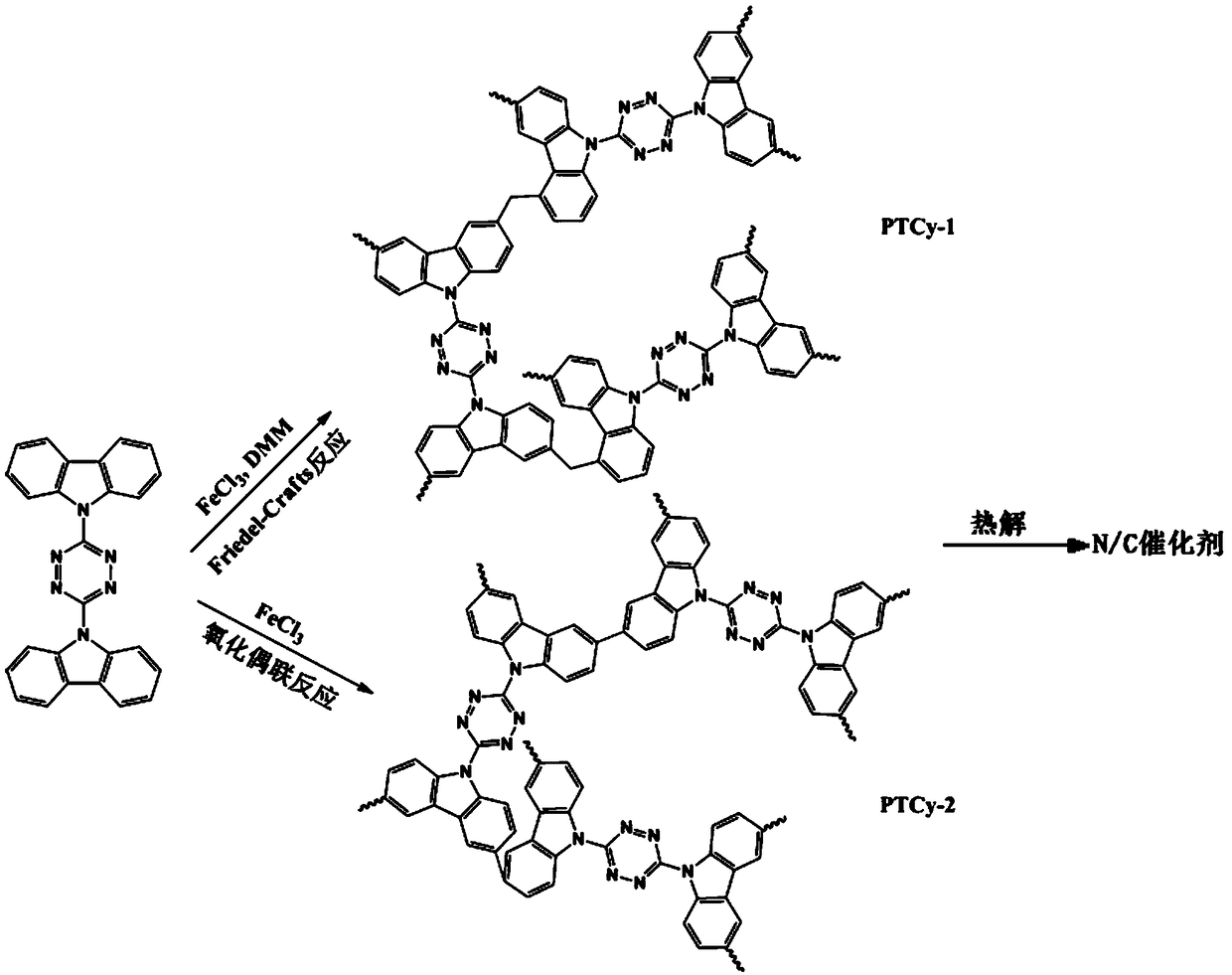
[0066] A nitrogen-doped carbon-oxygen reduction catalyst based on tetrazine ring polycarbazole, which is prepared by the following preparation method: first, 3,6-bis(3,5-dimethylpyrazole)-1,2 , 4,5-tetrazine prepared 3,6-bis(carbazolyl)-1,2,4,5-tetrazine (TCy) monomer by substitution reaction, and then 3,6-bis(carbazolyl) -1,2,4,5-Tetrazine (TCy) is obtained by Friedel-Crafts alkylation reaction to obtain tetrazine ring polycarbazole; then tetrazine ring polycarbazole is pyrolyzed to obtain tetrazine ring polycarbazole Nitrogen-doped carbon-oxygen reduction catalysts.
Embodiment 2
[0068]A nitrogen-doped carbon-oxygen reduction catalyst based on tetrazine ring polycarbazole, which is prepared by the following preparation method: first, 3,6-bis(3,5-dimethylpyrazole)-1,2 , 4,5-tetrazine prepared 3,6-bis(carbazolyl)-1,2,4,5-tetrazine (TCy) monomer by substitution reaction, and then 3,6-bis(carbazolyl) -1,2,4,5-tetrazine (TCy) can be obtained tetrazine ring polycarbazole through oxidative coupling reaction; then tetrazine ring polycarbazole is pyrolyzed to obtain tetrazine ring polycarbazole-based nitrogen-doped Heterocarbon oxygen reduction catalyst.
Embodiment 3
[0070] A method for preparing a nitrogen-doped carbon-oxygen reduction catalyst based on tetrazine ring polycarbazole, comprising the following steps:
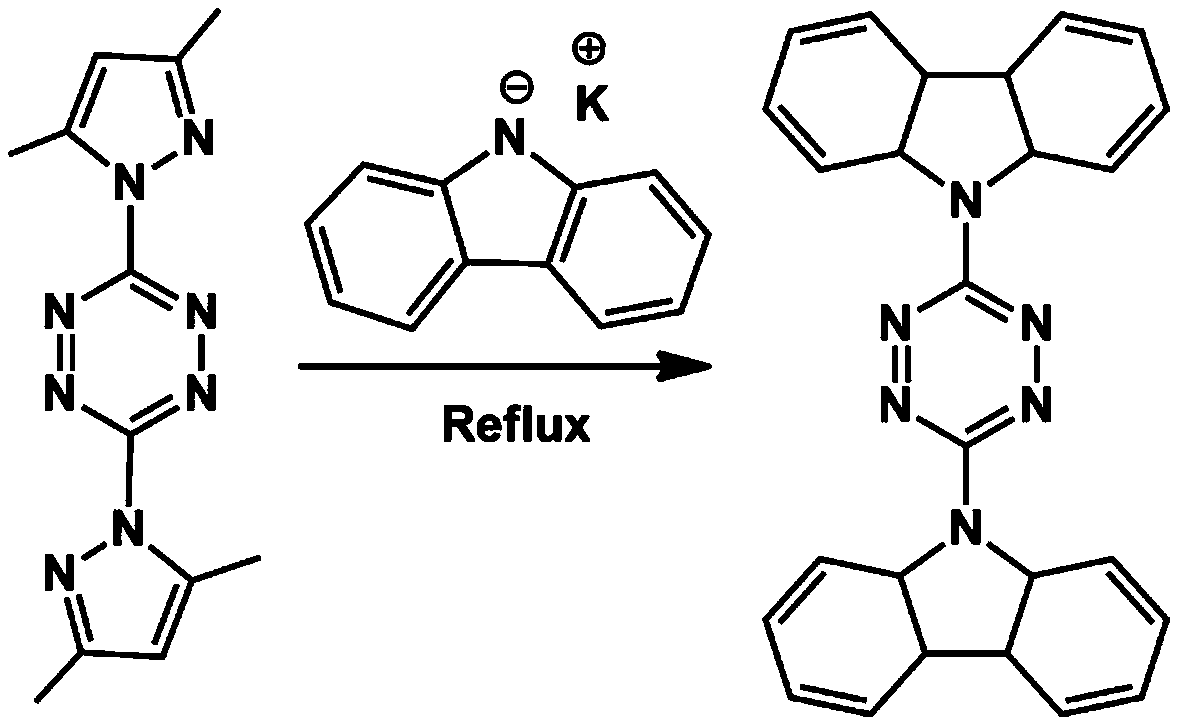
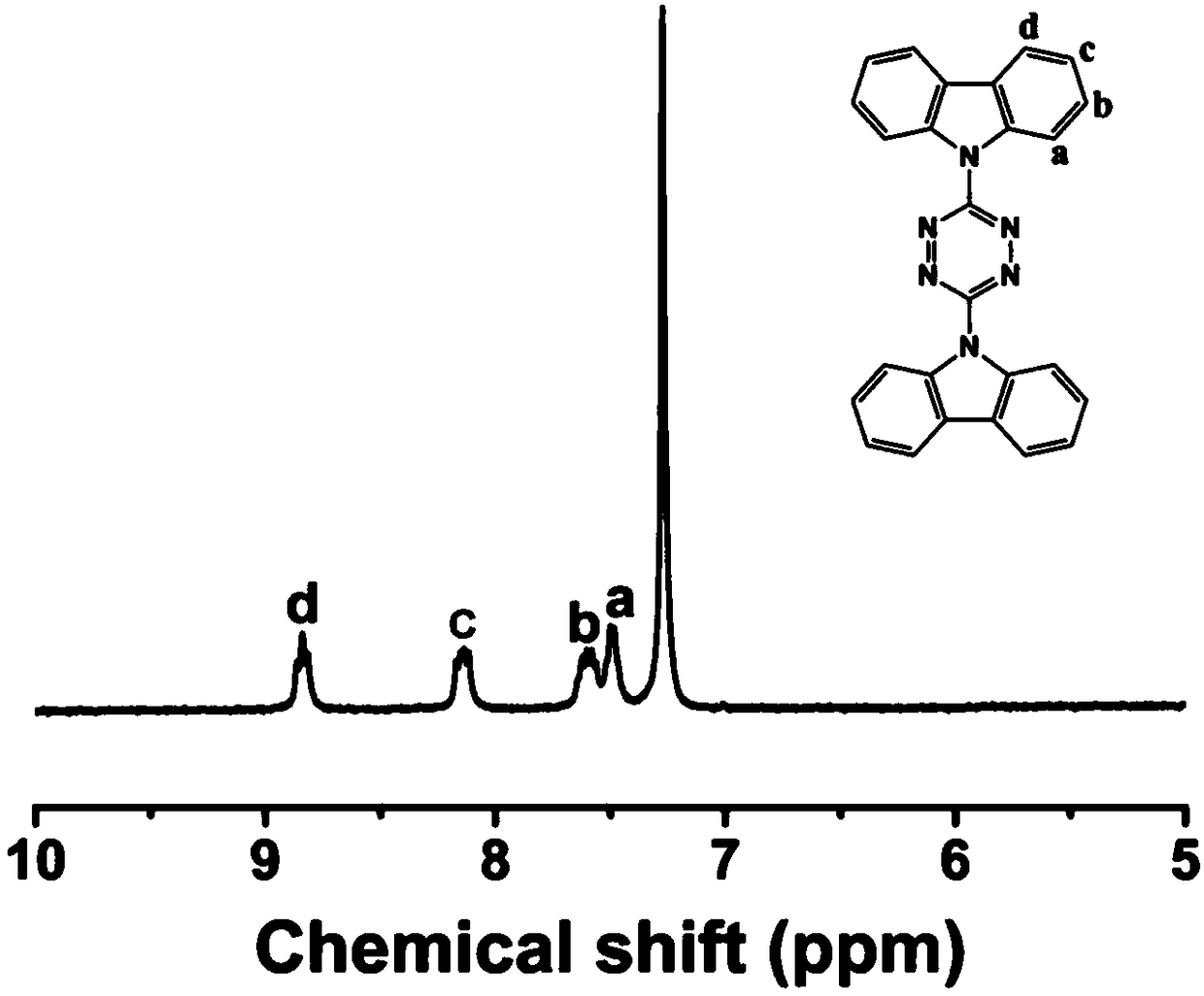
[0071] (1) Preparation of 3,6-bis(carbazolyl)-1,2,4,5-tetrazine (TCy): add 10mmol 3,6-bis(3,5-dimethylpyrazole) -1,2,4,5-tetrazine suspension in acetonitrile was added to the flask with 22mmol carbazole salt (preparation method: carbazole was added KOH at 0°C, stirred for 5h), stirred at room temperature for one hour, refluxed for 8h; cooled to At room temperature, filter, wash with acetonitrile and dry, and finally pass through the column with petroleum ether / chloroform; figure 2 is the composite map of TCy, image 3 is the NMR map of TCy;
[0072] (2) Synthesis based on 1,2,4,5-tetrazine ring polycarbazole hypercrosslinked polymer network (PTCy-1): add 40mmol dimethoxymethane to the nitrobenzene solution system of 10mmolTCy, stir 30min, then 40mmol FeCl 3 Added to the reaction system, and reacted at 45°C for 5h, and rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com