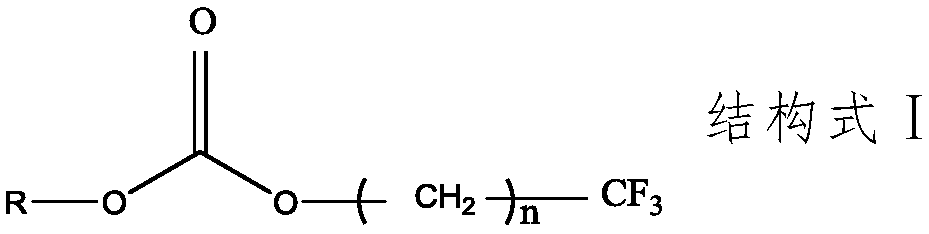Electrolyte additive, lithium battery electrolyte and lithium ion battery
An electrolyte additive and electrolyte technology, applied in the field of lithium batteries, can solve the problems of low chemical stability window, reduced cycle performance and safety performance of lithium ion batteries, etc., to widen the working voltage range, improve the electrochemical stability window, and improve stability. sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
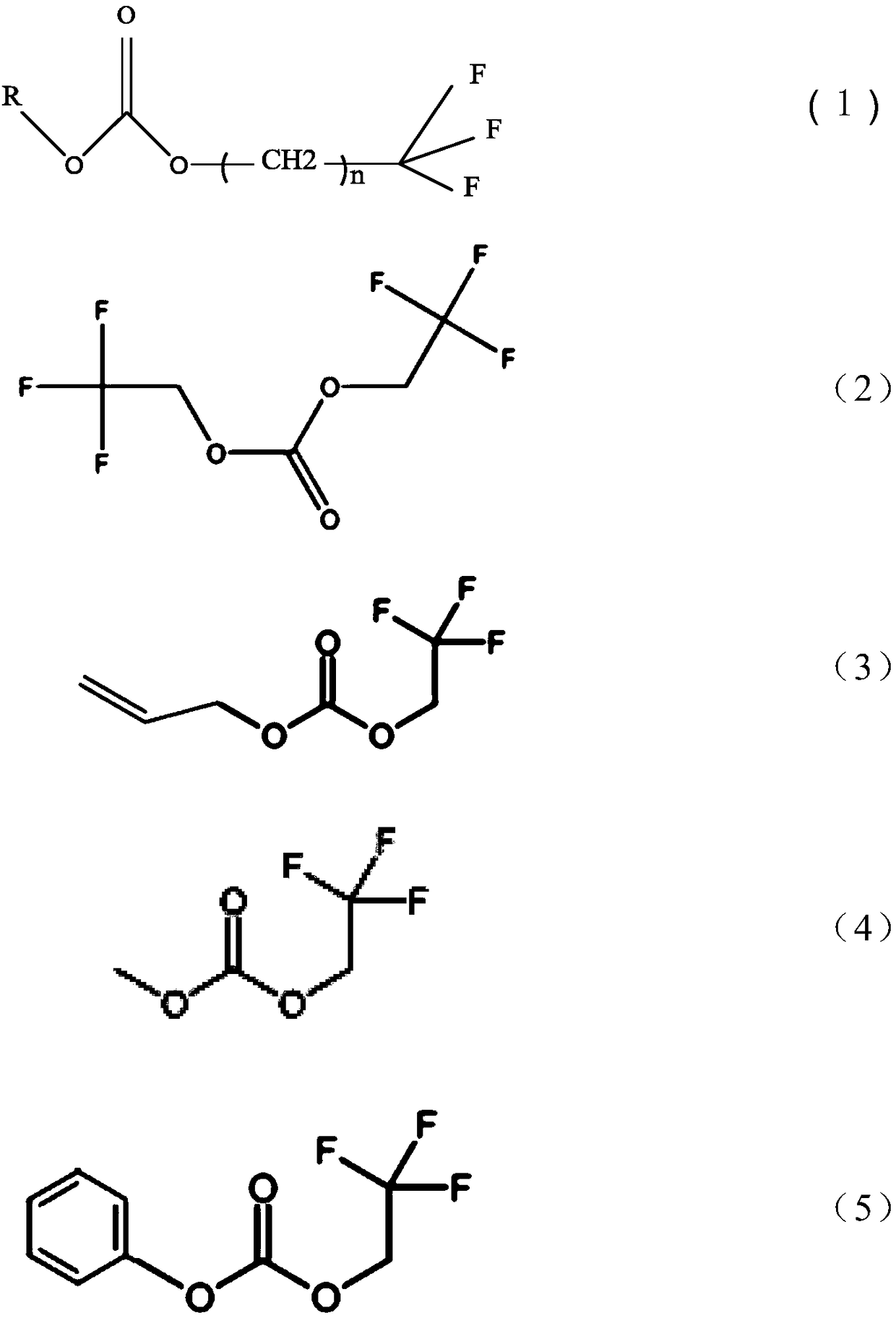
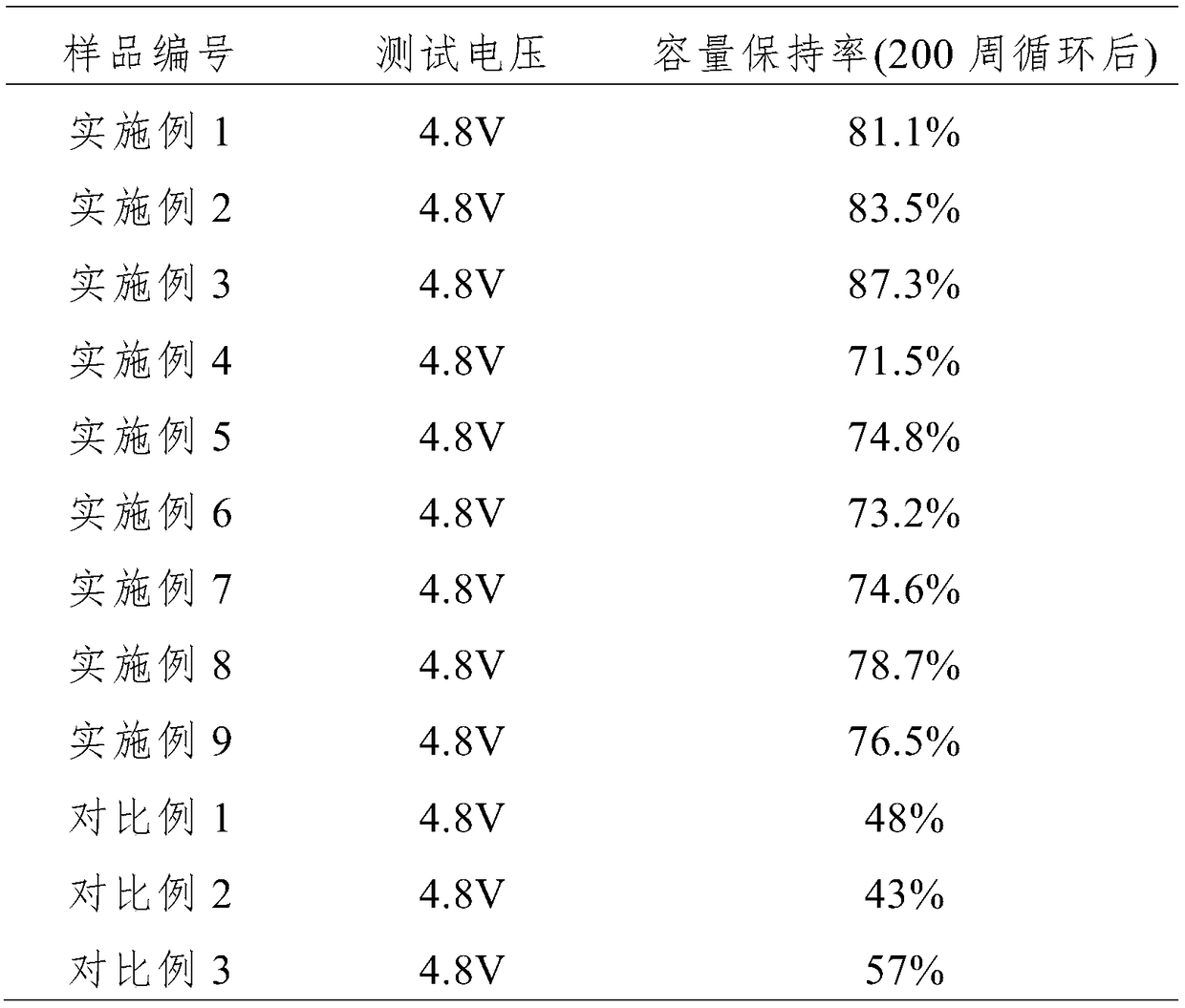
[0042] Dissolving bis(2,2,2-trifluoroethyl)carbonate accounting for 1% of the mass percentage of the basic electrolyte as an electrolyte additive in the basic electrolyte, wherein the organic solvent of the basic electrolyte is ethylene carbonate, Propylene carbonate, diethyl carbonate and dimethyl carbonate are a mixture of 1:1:3:3 by mass ratio EC:PC:DEC:DMC, and lithium salt LiPF is added 6 , its molar concentration is 1mol / L, with LiNi 0.8 co 0.1 mn 0.1 o 2As the positive electrode active material, graphite is used as the negative electrode active material. The positive and negative electrode current collectors are made of aluminum foil and copper foil respectively, and the diaphragm is made of ceramic diaphragm. The test battery is assembled into a ternary / graphite button battery. The test temperature is carried out at room temperature.
Embodiment 2
[0044] This example is based on Example 1, the difference is that, as an additive, 3% bis(2,2,2-trifluoroethyl)carbonate is dissolved in the mass ratio of EC :PC:DEC:DMC=1:1:3:3,LiPF 6 In the basic electrolyte with a concentration of 1.2mol / L.
Embodiment 3
[0046] This example is based on Example 1, the difference is that 5% by mass percentage of bis (2,2,2-trifluoroethyl) carbonate as an additive, dissolved in the mass ratio of EC :PC:DEC:DMC=1:1:3:3,LiPF 6 In the basic electrolyte with a concentration of 1.2mol / L.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com