A kind of aqueous electrolyte and aqueous metal ion battery
A water-based electrolyte, metal ion technology, applied in water-based electrolytes, secondary batteries, circuits, etc., can solve the problems of material dissolution, low capacity retention or Coulomb efficiency, and achieve high capacity retention and stable electrode/electrolyte interface. , the effect of good electrical conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 1.1 Preparation of aqueous electrolyte
[0048] 3.64g triethyl phosphate, 2.12g LiClO 4 (anhydrous) and 0.018gH 2 O mixed (stabilizer: water: metal salt molar ratio = 2:0.1:2), heated and stirred at 80°C, and allowed to cool to room temperature to obtain an aqueous electrolyte solution.
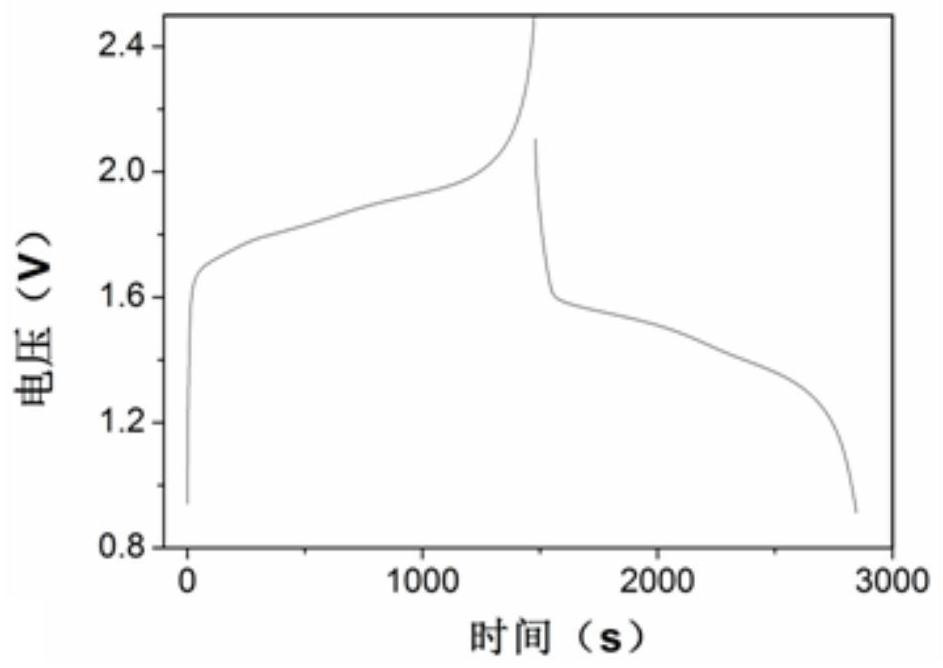
[0049] 1.2 Performance test
[0050] (1) Electrochemical window test
[0051] The glassy carbon electrode was used as the working electrode, the platinum wire was used as the counter electrode, and the silver-silver chloride was used as the reference electrode, and the test was carried out as a three-electrode system.
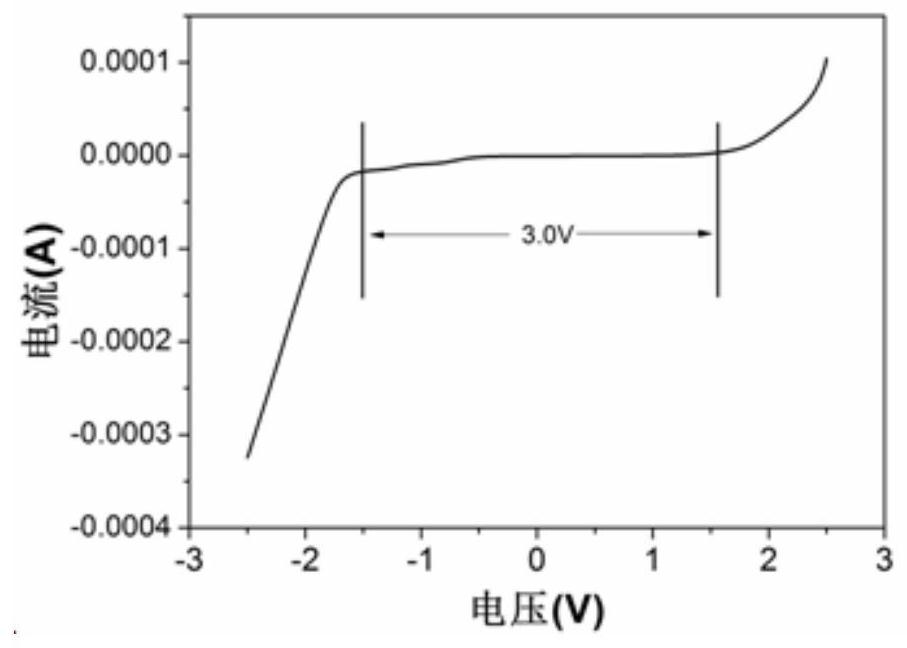
[0052] The above-mentioned three-electrode system was subjected to a cyclic voltammetry test on a Sultron (Salartron Analytical 1470E electrochemical test system in the UK), with a voltage range of -2.5V to 2.5V and a scan rate of 20mV / s.
[0053] For test results see figure 1 , figure 1 From the cyclic voltammetry curve obtained in Example 1 of the present invent...
Embodiment 2~3
[0059] 1.1 Preparation of aqueous electrolyte
[0060] Prepare the aqueous electrolyte according to the preparation process of Example 1, the difference is that triethyl hemiphosphate is replaced by equimolar acetone; that is, the stabilizer is a mixture of triethyl phosphate and acetone, and the mixture of triethyl phosphate and acetone Molar ratio is 1:1, is recorded as embodiment 2.
[0061] The aqueous electrolyte solution was prepared according to the preparation process of Example 1, except that triethyl phosphate was replaced by acetone, which was recorded as Example 3.
[0062] 1.2 Performance test
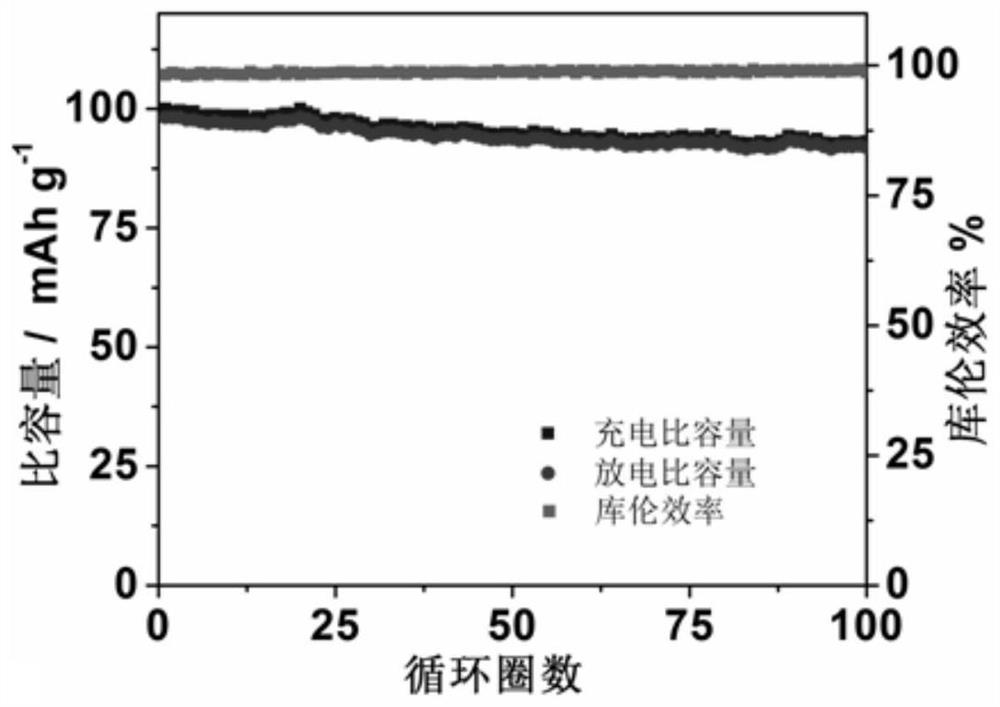
[0063] According to the test method of Example 1, the performance tests of Examples 2-3 were carried out respectively, and compared with Example 1, the results are shown in Table 1.
[0064] The performance test result of table 1 embodiment 2-6
[0065] Electrochemical window, V Charge and discharge voltage, V Capacity retention Coulombic efficiency ...
Embodiment 4~5
[0069] 1.1 Preparation of aqueous electrolyte
[0070] Prepare the aqueous electrolyte solution according to the preparation process of Example 1, the difference is that the amount of water is increased to make the molar ratio of stabilizer: water: metal salt = 2:1:2; record it as Example 4.
[0071] The aqueous electrolyte was prepared according to the preparation process of Example 1, except that the amount of stabilizer was reduced so that the molar ratio of stabilizer: water: metal salt = 1:0.1:2; recorded as Example 5.
[0072] 1.2 Performance test
[0073] According to the test method of Example 1, the performance tests of Examples 4-5 were carried out respectively, and compared with Example 1, the results are shown in Table 2.
[0074] The performance test result of table 2 embodiment 7-8
[0075] Electrochemical window, V Charge and discharge voltage, V Capacity retention Coulombic efficiency Example 1 3.0 2.5 94.6% 99.2% Example 4 2.5 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com