Therapeutic protein-loaded nanoparticle and method for preparing the same
A therapeutic and protein technology, which is applied in the field of preparation of pharmaceutical compositions, pharmaceutical preparations containing the nanoparticles, suspensions or pharmaceutical compositions, and nanoparticles loaded with therapeutic proteins, which can solve the problem of controllability and stability And problems such as unsatisfactory repeatability, uneven particle size distribution, and large nanoparticle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] Preparation of Nanoparticles of Example 1 Loaded Insulin
[0128] 1. Preparation process:
[0129] (1) Dissolving insulin in a hydrochloric acid solution with a pH of 2.8 to obtain an insulin solution with a concentration of 0.5 mg / mL.
[0130] (2) Chitosan (90KDa, 85% deacetylation) was dissolved in 0.2% acetic acid solution to obtain a 1 mg / mL chitosan solution, and then the pH was adjusted to 5.3 with NaOH solution.
[0131] (3) Sodium tripolyphosphate was dissolved in 0.025M HEPES buffer to obtain a 0.2 mg / mL sodium tripolyphosphate solution.
[0132] (4) Put chitosan solution, sodium tripolyphosphate solution, insulin solution and double-distilled water into four syringes respectively, place the four syringes on the high-pressure pump respectively, and the injection holes of each syringe are connected with the plastic tube 1 One end of each of -4 is airtightly connected, and the other end of the plastic pipe is airtightly connected with the four passages of the f...
Embodiment 2
[0144] Example 2. Particle size test, potential test and morphological characterization:
[0145] 1. Particle size test:
[0146] The particle size and polydispersity index (PDI) of the nanoparticles in the suspension were measured using a Malvern particle sizer (with a dynamic light scattering detector).
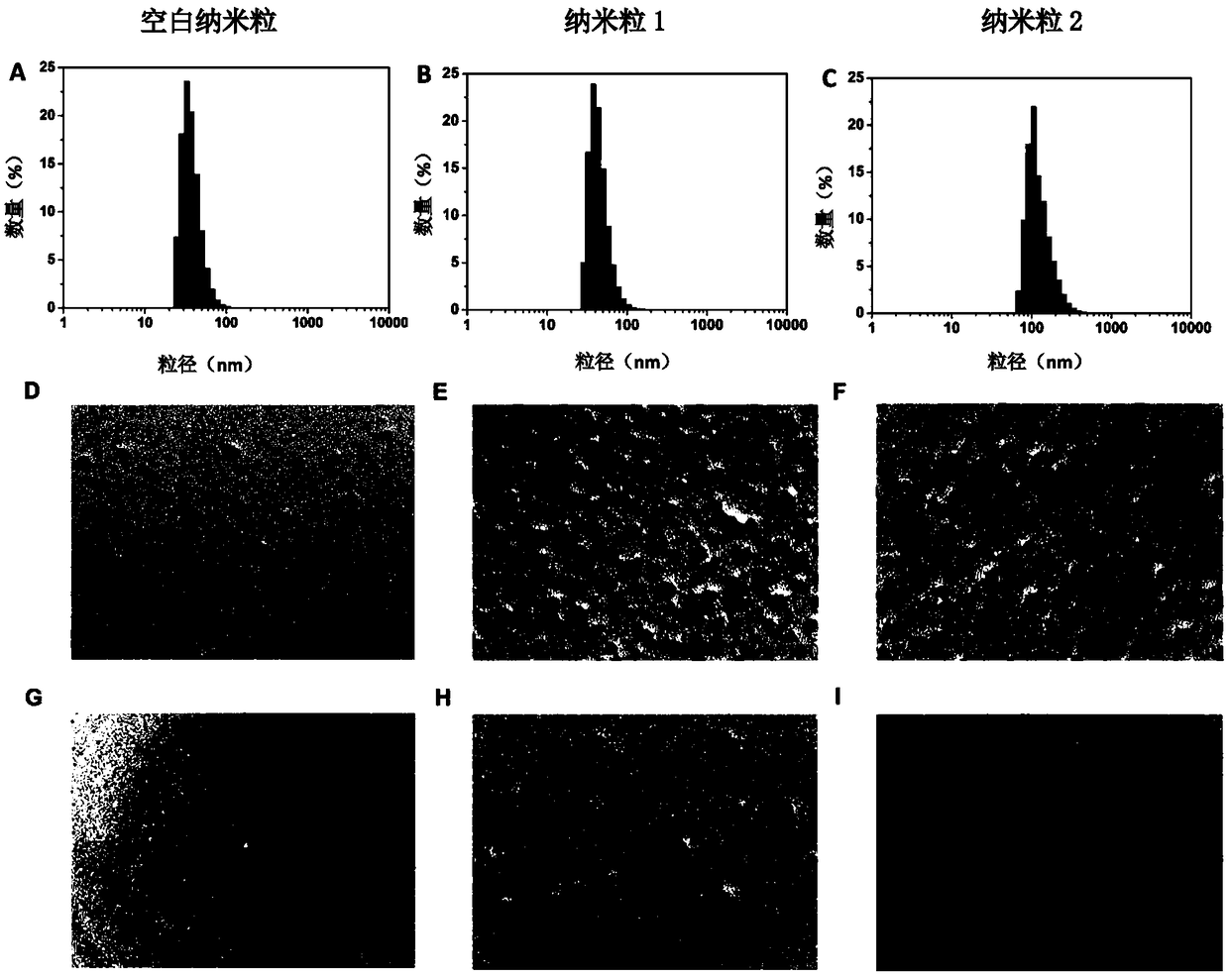
[0147] image 3 A, B, and C are the test results of blank nanoparticles, nanoparticles 1, and nanoparticles 2, respectively, using a Malvern particle size analyzer. The average particle diameters of blank nanoparticles, nanoparticles 1 and nanoparticles 2 were 37.7nm, 45.4nm and 117.7nm, respectively, and the PDIs of nanoparticles 1 and nanoparticles 2 were 0.139 and 0.146, respectively. The results show that the insulin-loaded nanoparticles prepared by the method of the present invention have small particle size and narrow particle size distribution, and compared with the insulin-free nanoparticles prepared under the same conditions, the particle size difference is not l...
Embodiment 3
[0161] The calculation of embodiment 3 encapsulation efficiency and drug load
[0162] The suspension containing nanoparticle 1 was ultrafiltered at 3000rpm for 20min, and then the ultrafiltrate was taken to measure the UV absorbance, and compared with the standard insulin sample, the encapsulation efficiency and drug loading capacity of the nanoparticle were calculated according to the following formula:
[0163] Encapsulation efficiency=(total drug amount-free drug amount) / total drug amount×100%;
[0164] Drug loading = total amount of drug in nanoparticles / total amount of nanoparticles × 100%.
[0165] It is calculated that the encapsulation efficiency of the nanoparticle 1 is 91%, and the drug loading is 27.5%.
[0166] Three kinds of sodium tripolyphosphate solutions with different pH were used for preparation, the pHs of the obtained suspensions were 6.0, 6.2 and 6.5 respectively, and the encapsulation efficiencies of nanoparticles in the suspensions were 65%, 80% and 9...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com