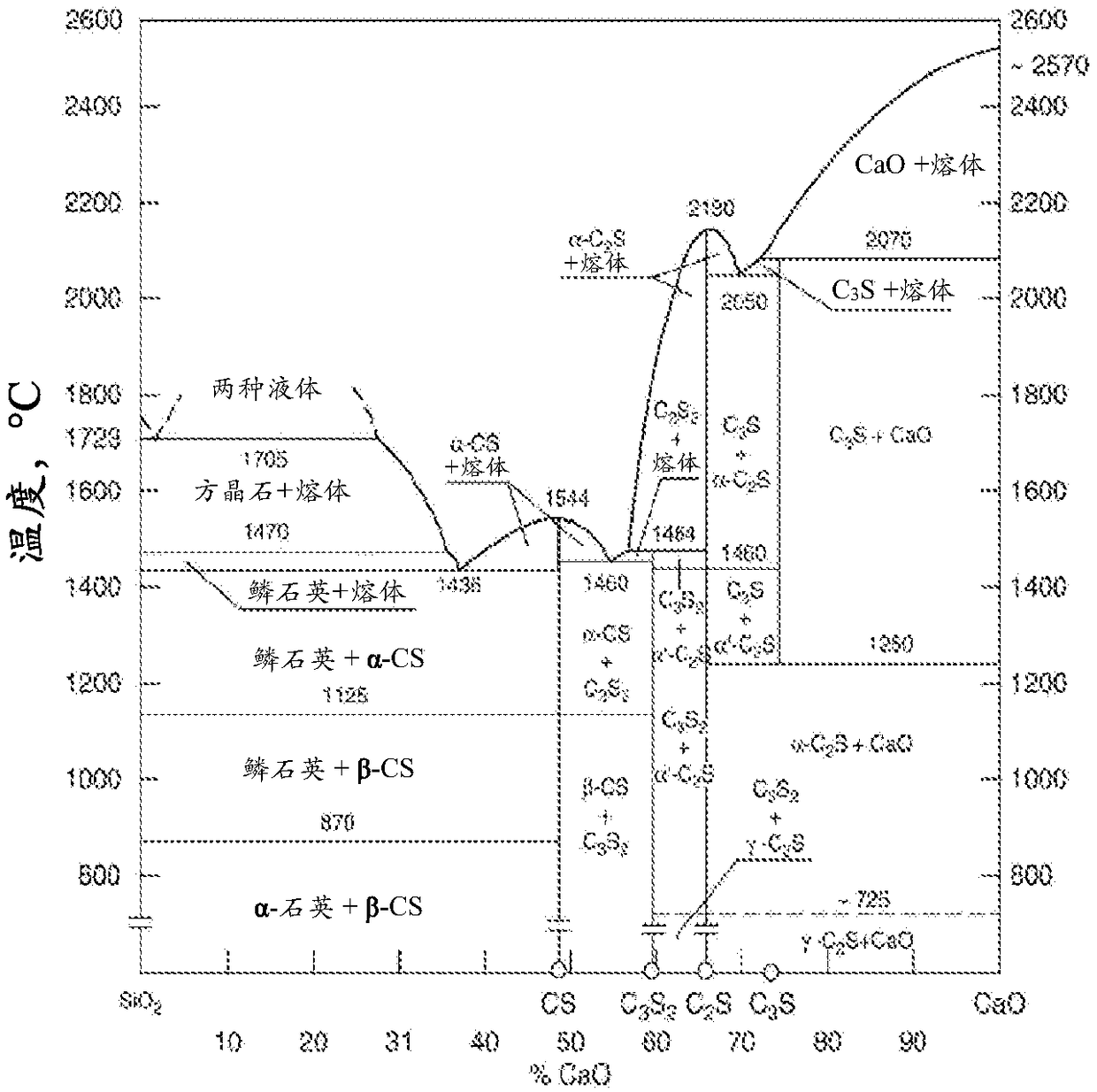
Methods for low energy inorganic material synthesis
An inorganic and reaction medium technology, applied in the field of synthesis of low-energy inorganic materials, can solve problems such as limitations in versatility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
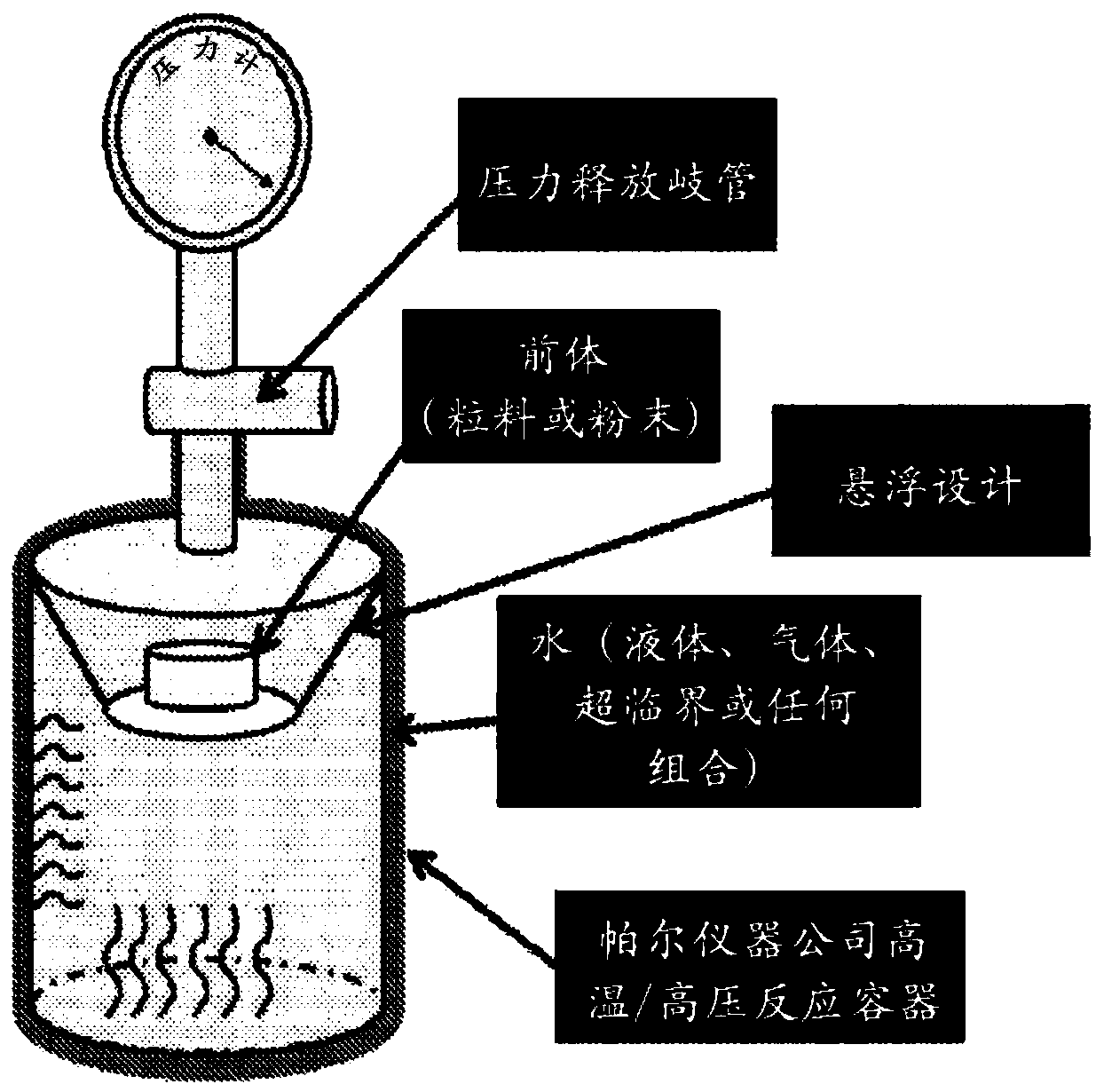
[0179] Calcium carbonate (3.75 g, Acros 98%, CAS#471-34-1) and silicon dioxide (2.25 g, quartz, US Silica Co.) in an equimolar ratio (1:1), Berkley Springs, WV (CAS#14808-60-7) in Spex mill (8000M Mixer / Mill SpexCertiprep, zirconia vials and media, Metuchen, NJ) ) for 10 minutes and then pressed into 1" cylindrical pellets (96.8 MPa). The pellets were then suspended in a Hastalloy C-276 alloy hydrothermal reaction vessel (HAST C 1807H04, Parr Instruments ), above a deionized (18.2 MΩ) liquid water bath in Moline, IL), as figure 2 shown.
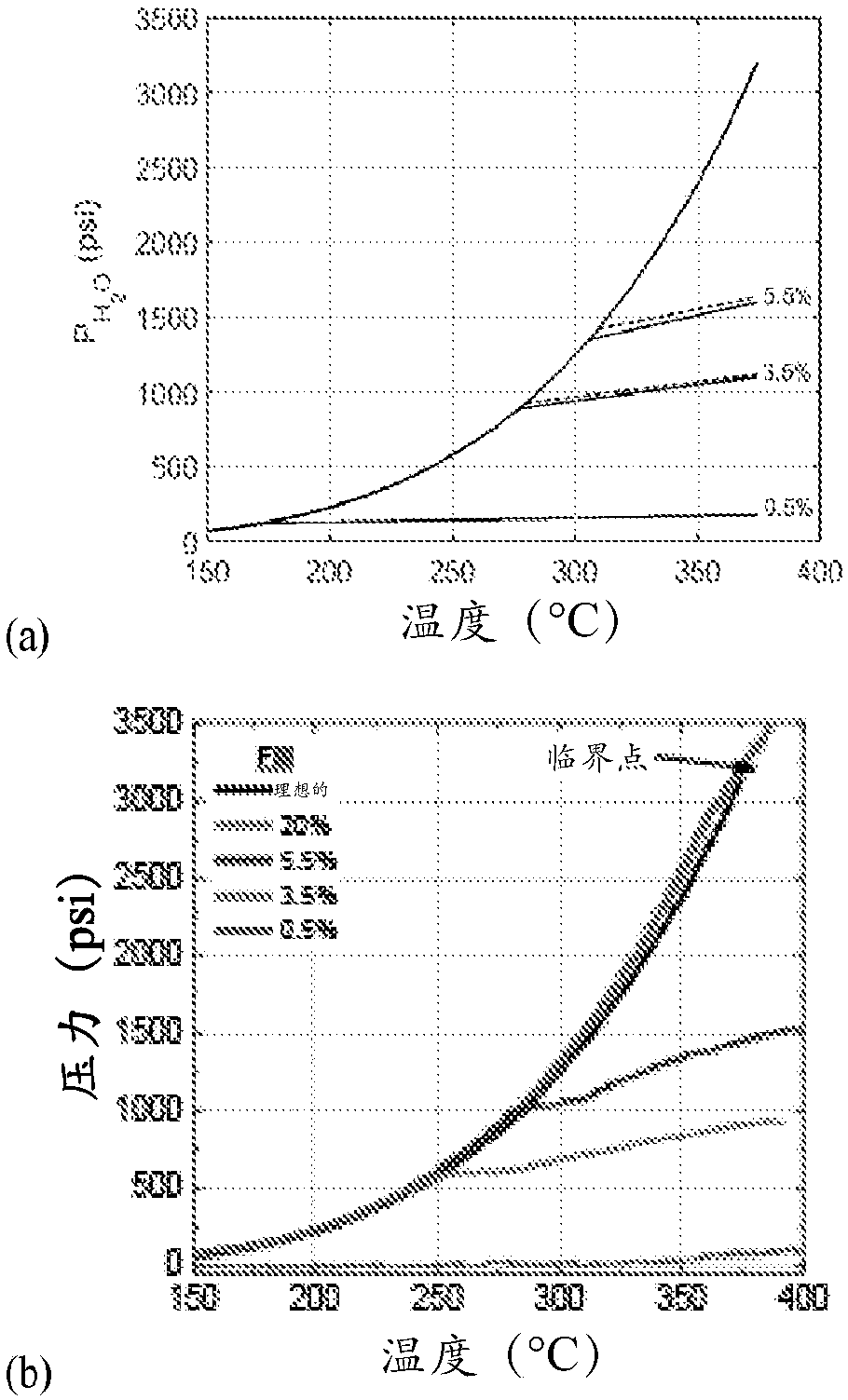
[0180] Since the amount of water determines the pressure generated, the percent volume fill varies from 0 to 20%. The Vander-Waals equation is used to numerically calculate the resulting pressure:
[0181]
[0182] where P is the pressure (atm), V is the volume (liter), n is the amount of water (mol), R is the molar gas constant (8.314J / mol·K), and a and b are constants of proportionality (for H 2 O, a=5.46atm·L 2 / mol 2 , and b=0.0...
example 2
[0193] Analysis of CaSiO empirically 3 Synthetic Kinetics. 1:1 CaCO 3 :SiO2 2 The mixture was subjected to specific hydrothermal reaction conditions (described elsewhere, in the main manuscript) for indicated periods of time, and then characterized by x-ray diffraction (XRD) and thermogravimetric analysis (TGA). The reaction time is defined as the time after which stable pressure and temperature are reached ( Figure 18 ). Using this definition, the following reaction times were chosen: 0.5, 1.5, 6, 8.5, 14 and 63 h. XRD was used to confirm the crystallinity, while TGA was used to quantify the reaction yield. Since no CaO phase appears in the XRD pattern, the remaining CaCO in the product 3 The decomposition of is satisfactory for accurate quantification. If the reaction proceeds to 100%, the mass loss in the region of 650°C-800°C will be 0 wt%. Alternatively, by dividing the mass loss in this region by the theoretical CO in the unreacted sample 2 amount to determine ...
example 3
[0197] about Further studies of the reactions include optimization of kinetics. Using a method similar to that of Zhang et al. (J. Am. Ceram. Soc. 5, 2015), we incorporated sodium chloride (NaCl) into the raw material mixture to enhance diffusion. The exact procedure for adding NaCl is as follows:
[0198] (1) A 30 wt% NaCl solution was generated by mixing reagent grade NaCl with deionized water.
[0199] a. Once all the NaCl is dissolved, the solution is considered ready for the experiment
[0200] (2) Add 5 grams of 1:1 CaCO 3 :SiO2 2 The mixture was mixed with 5 g of the NaCl solution
[0201] (3) Dry the mixture overnight at 90°C
[0202] (4) Compress 1" pellets with a pressure of 96 MPa.
[0203] (5) The pellets were then reacted at 430° C. and 30 ml (approximately 850 psi) for the specified amount of time.
[0204] Results from this study are also depicted along with conventional kinetic studies in Figure 19 middle. The addition of NaCl increased the kinetics...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| process yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com