Cu-based catalyst precursor and method for controlling its crystal phase crystallinity and Cu-based catalyst and preparation method thereof
A catalyst and precursor technology, applied in the field of catalyst preparation, can solve the problem of effectively adjusting the precursor, etc., which is not mentioned, and achieves the effects of convenient operation, simple process and increased specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Dissolve the mixture containing 2.2kg of copper nitrate, 1.0kg of zinc nitrate, 0.8kg of aluminum nitrate and 0.07kg of magnesium nitrate in 5.5L of deionized water to make a 7.5L aqueous solution, which is referred to as solution A;
[0063] Dissolve 1.6kg of sodium carbonate in 6.8L of deionized water to make a 7.5L solution, which is referred to as solution B;
[0064] Preheat solution A and solution B to 70°C for use.
[0065] Under stirring, the solution A and the solution B were co-precipitated, the temperature was controlled at 80°C during the precipitation process, and the pH value at the end of the precipitation was controlled at 8. The precipitated suspension was aged at 80° C. for 4 h, and then filtered through a Buchner funnel to obtain a catalyst precursor.
Embodiment 1-1
[0067] Wash the catalyst precursor prepared in Example 1 with water until the conductivity is less than 50μs / cm, collect the filter cake and place it in a 250mL crucible, then directly transfer it to an oven, set the drying temperature of the oven to 110°C, and dry at this temperature 18h. Then the dried catalyst precursor was crushed into particles of about 2 mm and transferred to a muffle furnace for calcination at a temperature of 400° C. for 3 h. Ready to use after molding.
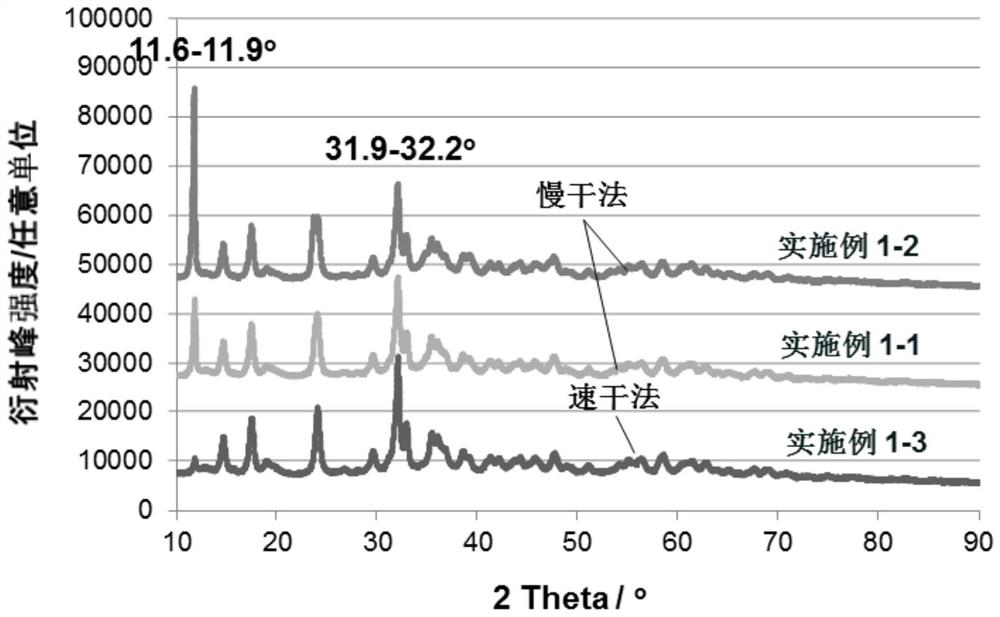
[0068] in, figure 1 shows the XRD pattern of the catalyst prepared by the slow drying method of this embodiment. The diffraction peak at 11.6-11.9° is the main peak of hydrotalcite, crystal plane (003), and the diffraction peak at 31.9-32.2° is the main peak of copper-zinc malachite, a mixture.
Embodiment 1-2
[0070] The catalyst precursor prepared in Example 1 was washed with water until the conductivity was less than 50 μs / cm, and 10 min of ultrasonic vibration (frequency of 40 kHz) was added during the washing and beating process; the filter cake was collected and placed in a 250 mL crucible, and then directly transferred to Oven, set the oven drying temperature to 110°C, and dry at this temperature for 18 hours. Then the dried catalyst precursor was crushed into particles of about 2 mm and transferred to a muffle furnace for calcination at a temperature of 400° C. for 3 h. Ready to use after molding.
[0071] in, figure 1 shows the XRD pattern of the catalyst prepared by the slow drying method of this embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com