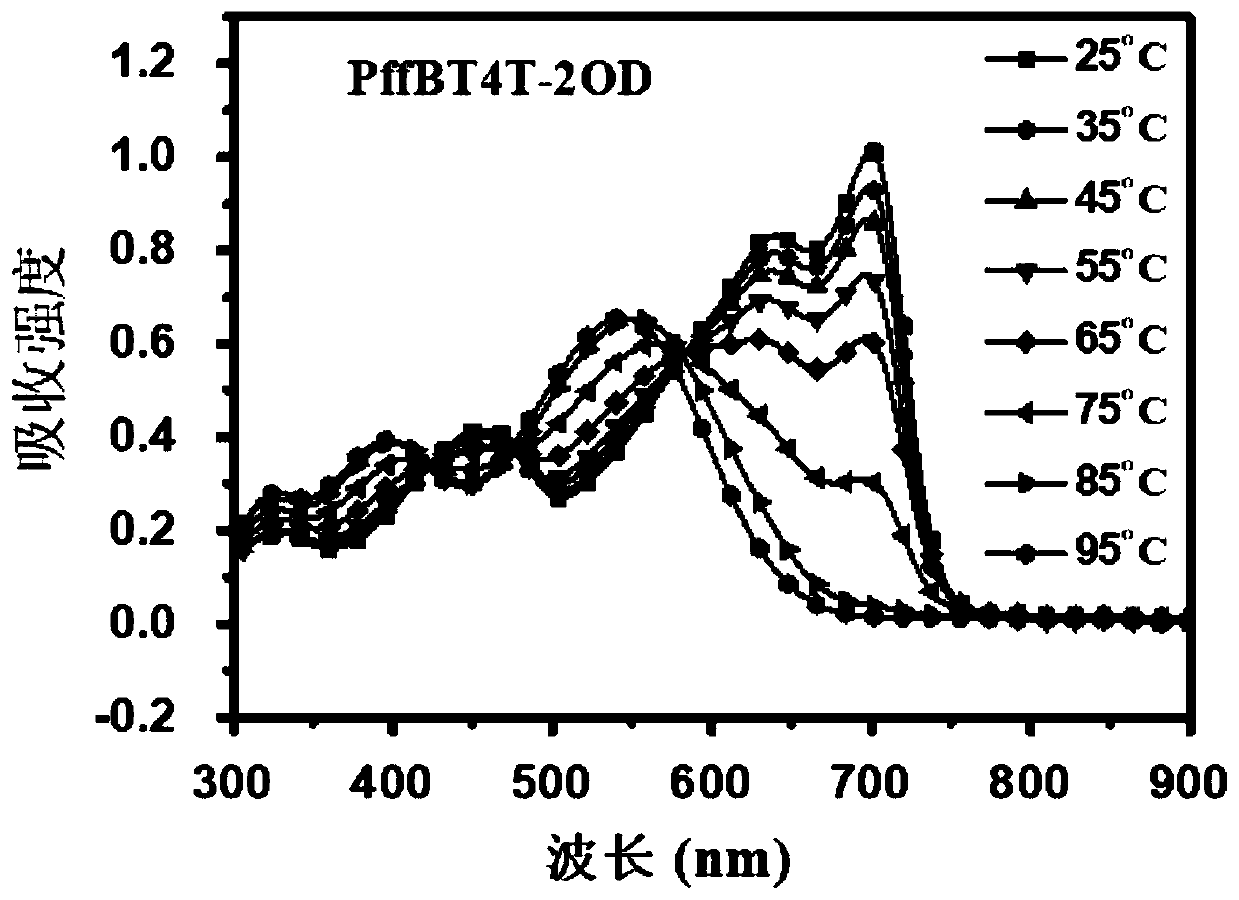
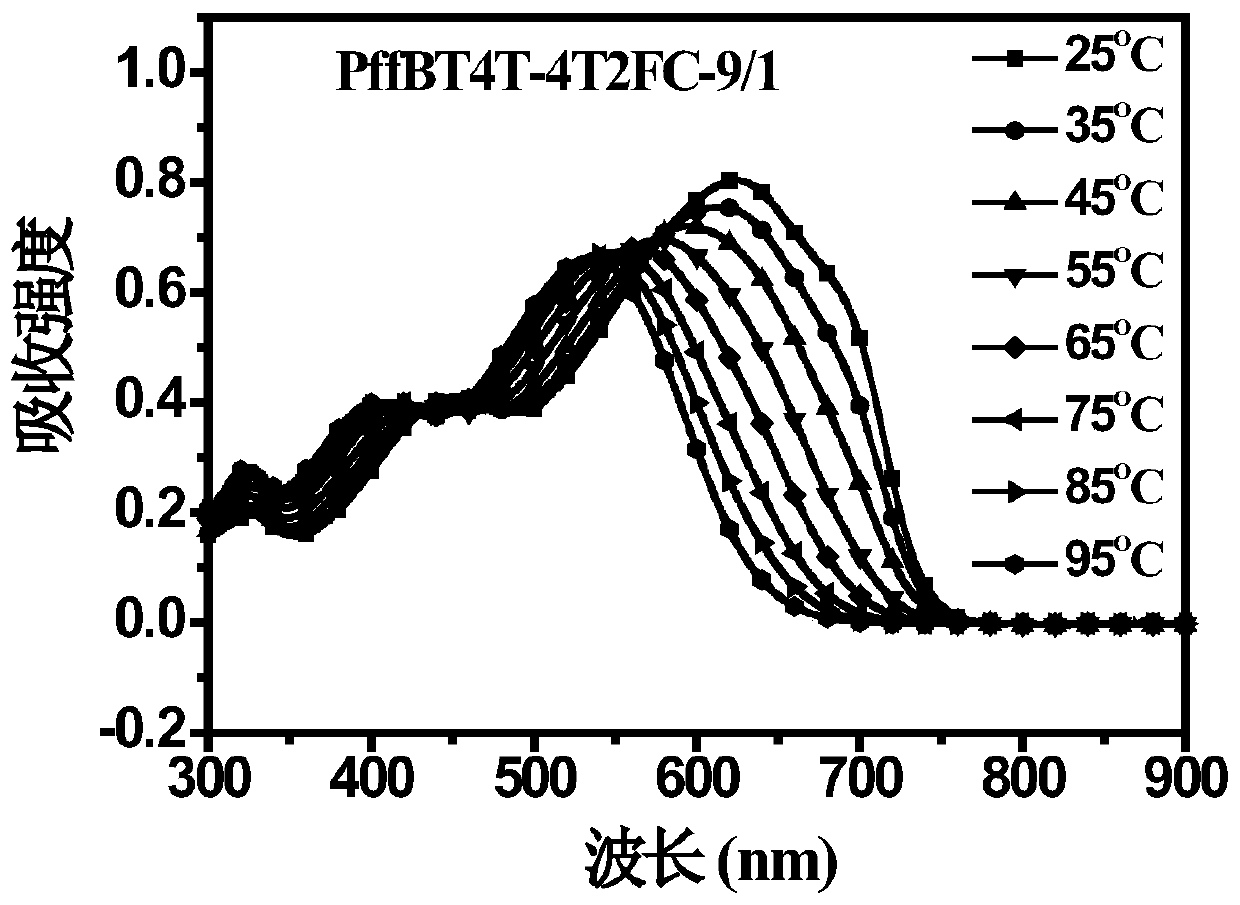
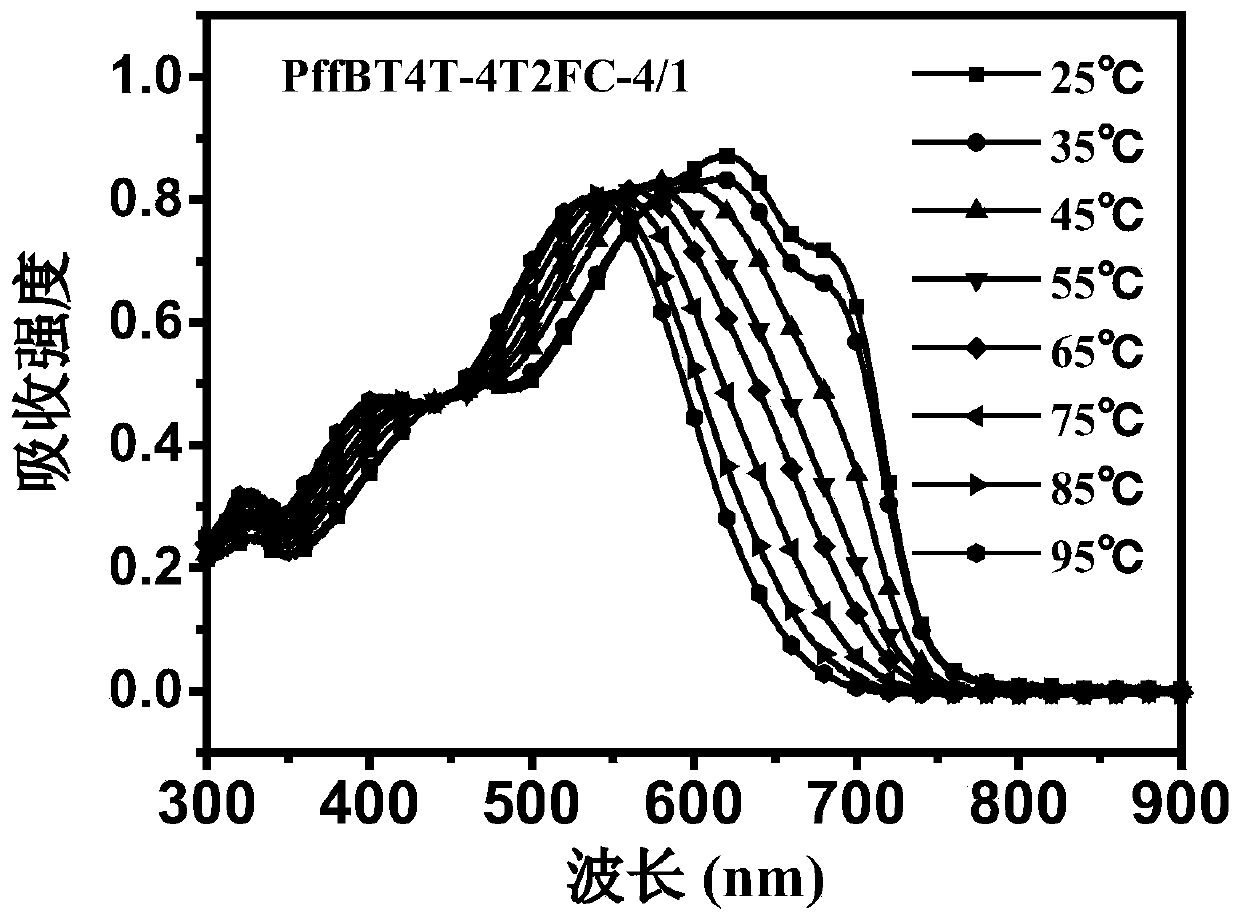
Polymer donor with reduced crystallinity, and preparation method and application thereof
A crystalline, polymer technology, applied in the field of chemistry, can solve the problems of low filling factor, scarcity, disadvantage, etc., and achieve the effects of good phase morphology, high device efficiency, and strong feasibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1. Synthesis of PffBT4T-4T2FC-9 / 1.
[0034]
[0035] a) 2-Bromo-3-thiophenecarboxylic acid (2.64g, 12.74mmol), 1-bromo-2-hexyldecane (11.68g, 38.02mmol) and potassium carbonate (8g, 57.88mmol) were added to a round bottom flask, The reactant was dissolved with 30ml DMF as solvent. Stir at 80°C for 40 hours. After the reaction was completed, it was cooled to room temperature. Pour the mixture into a pear-shaped separatory funnel, and 2 Cl 2 After extraction, the obtained organic phase was dried over anhydrous sodium sulfate. After evaporating the solvent, the column was passed to obtain a total of 4.94 g of pure compounds, with a yield of 90%;
[0036] b) The product of step a) (2 g, 4.64 mmol), (3,3'-difluoro-[2,2'-bithiophene]-5,5'-diyl)bis(trimethylstannane) ( 0.96g, 1.8mmol) and tetrakis (triphenylphosphine) palladium (170mg, 0.13mmol) were packed into a double-necked reaction flask, and after nitrogen was replaced three times with an oil pump, anhy...
Embodiment 2
[0040] Example 2. Synthesis of PWBT4T-4T2FC-4 / 1.
[0041]
[0042] a) 2-Bromo-3-thiophenecarboxylic acid (2.64g, 12.74mmol), 1-bromo-2-hexyldecane (11.68g, 38.02mmol) and potassium carbonate (8g, 57.88mmol) were added to a round bottom flask, The reactants were dissolved using 30ml DMF as solvent. Stir at 80°C for 40 hours. After the reaction was completed, it was cooled to room temperature. Pour the mixture into a pear-shaped separatory funnel, and 2 Cl 2 After extraction, the obtained organic phase was dried over anhydrous sodium sulfate. After evaporating the solvent, the column was passed to obtain a total of 4.94 g of pure compounds, with a yield of 90%;
[0043] b) The product of step a) (2 g, 4.64 mmol), (3,3'-difluoro-[2,2'-bithiophene]-5,5'-diyl)bis(trimethylstannane) ( 0.96g, 1.8mmol) and tetrakis (triphenylphosphine) palladium (170mg, 0.13mmol) were packed into a double-necked reaction flask, and after nitrogen was replaced three times with an oil pump, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com