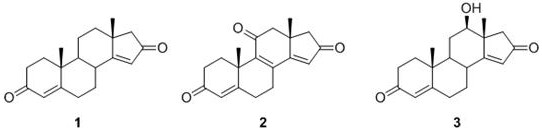
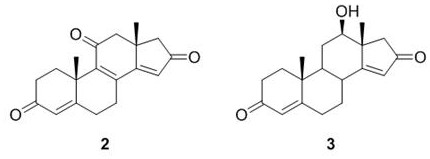
3,16-androstenediones and their applications
A kind of technology of androstenedione and compound, which is applied in the application field of preparing immunosuppressive and antitumor drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Preparation of 3,16-androstenediones
[0030] The samples of Simao vines were taken, dried and pulverized, and extracted twice with 70% ethanol solution by volume for 3 h each time. The filter residue was removed by filtration, and the extracts were combined, concentrated under reduced pressure, and then extracted with ethyl acetate. Extracted twice, concentrated to obtain an ethyl acetate layer extract; the ethyl acetate layer extract was roughly divided with macroporous adsorption resin D101, followed by pure water, 40%, 60%, 80%, 100% by volume A total of 5 fractions (Fr.A, Fr.B, Fr.C, Fr.D, Fr.E) were obtained by elution with aqueous methanol; 5 g of fraction D was used with C 18 The medium pressure column was washed with methanol aqueous solutions of 60%, 70%, 80%, and 90% by volume, and the 80% methanol water washed section was used to mix the samples with 100-200 mesh silica gel, and the petroleum ether-acetone two-phase system was used as the The mob...
Embodiment 2
[0041] Example 2: Immunosuppression detection test
[0042] (1) Preparation of spleen lymphocyte suspension
[0043] 18~22g healthy BABL / c mice were sacrificed by exsanguination, soaked in 75% alcohol for 5 minutes, taken out, placed in a sterile tray with the left side up, in a clean bench, and disinfected with sterilized Tweezers pick up the fur in the middle of the abdomen, make an incision, use another set of instruments to cut the layers of the abdominal wall, use a third set of instruments to take out the spleen, remove fat and connective tissue, put it in PBS (phosphate buffered saline), wash away Floating blood; then transfer the spleen tissue to a dish containing RPMI 1640 incomplete culture medium, cut it into small pieces with scissors, use a sterile syringe core to grind the spleen in a 200-mesh stainless steel mesh, and use PBS in small quantities several times Rinse, transfer the suspension to a 15 mL centrifuge tube with a pipette; centrifuge at 1000 r / min for ...
Embodiment 3
[0072] Example 3: In vitro antitumor activity test
[0073] (1) Materials
[0074]DMSO (Sigma, USA), fetal bovine serum (HyClone, USA), RPMI-1640 culture medium (HyClone, USA), phosphate buffer (Shanghai beyotime), double antibody (HyClone, USA), CCK-8 (East Ren Chemical Technology Co., Ltd.), human tumor cells (CCRF-CEM, MOLT-4, K-562, MALME-3M, UACC-62, SNB-75, OVCAR8, EKVX, U0-31, SF-295, NCI-H226 , SK-OV3, MDA_MB-468, Hop92), the compound of the present invention and dexamethasone were prepared with DMSO.
[0075] (2) method
[0076] Take five kinds of human tumor cells in the logarithmic growth phase and adjust the cell suspension to a certain concentration and inoculate them in a 96-well culture plate, 90 μl / well, culture for 24 hours, and then add different concentrations of compounds, 10 μl / well, each For each concentration, 3 replicate wells were set. Count the cells and measure the survival rate of the cells using the disc blue staining method, the survival rate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com