Method for synthesizing aminopyridine and 4-aminopyridine in one step
An aminopyridine and cyanopyridine technology, applied in the field of chemical raw material synthesis, can solve the problems of high condition requirements, many three-waste pollution, complicated steps and the like, and achieve the effects of easy control of conditions, few side reactions and simple reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
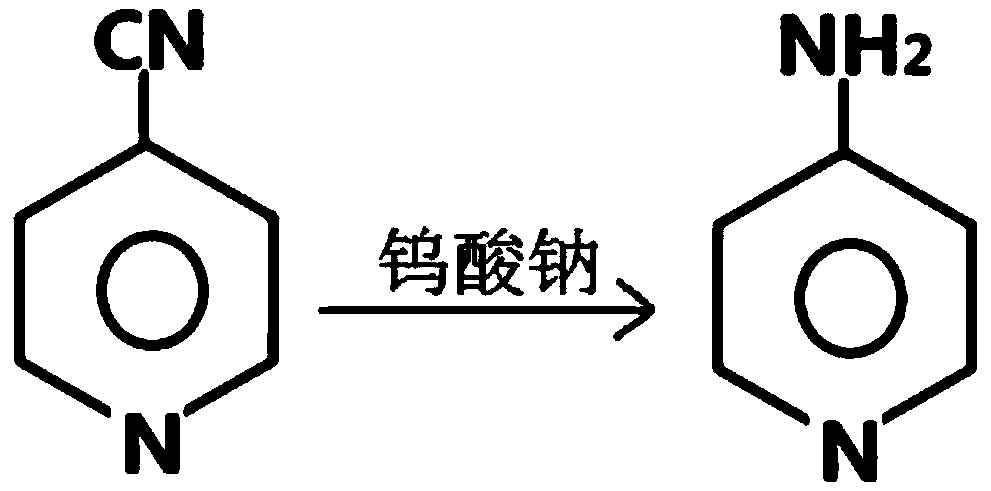
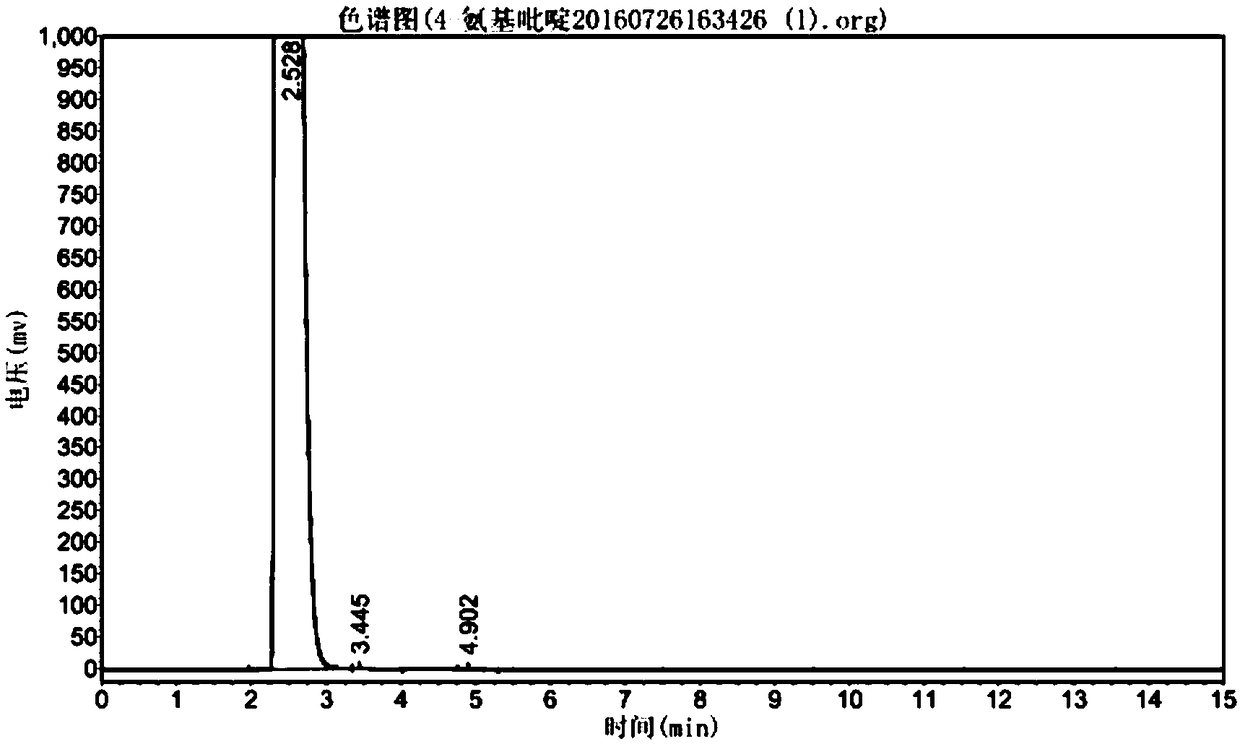
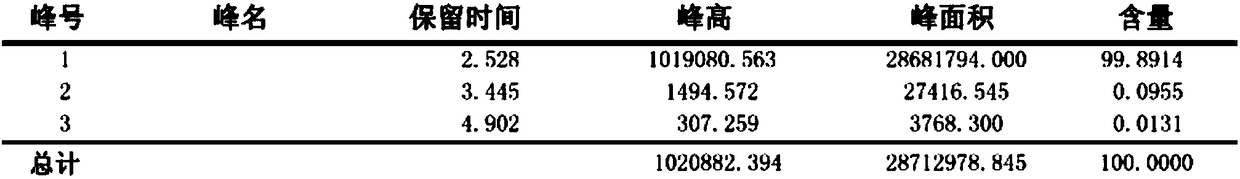
[0033] like figure 1 As shown, the present invention provides a method for synthesizing aminopyridine in one step. The main steps include: under low temperature conditions, using cyanopyridine as a raw material, using sodium tungstate as a catalyst, pre-reacting in an aqueous solution of sodium hypochlorite, and then heating up It is obtained by one-step Hoffman reaction. Compared with the prior art, the preparation method explored by the present invention has the advantages of easy control of reaction conditions, less by-products, less pollution of three wastes, etc., and is more suitable for industrial application. Taking 4-aminopyridine as an example, the above method is used to synthesize, and the main steps are:
[0034] (1) Prepare an aqueous solution of 4-cyanopyridine (in a reaction flask, dissolve 100 g of 4-cyanopyridine with 375 g of water to obtain an aqueous solution of 4-cyanopyridine), and cool the aqueous solution to below 0° C.;
[0035] (2) add catalyzer sod...
Embodiment 2
[0045] like figure 1 As shown, the method for the one-step synthesis of aminopyridine provided by the present invention, the main steps include: under low temperature conditions, using cyanopyridine as raw material, using sodium tungstate as catalyst (the addition of catalyst is 2% of the quality of raw material cyanopyridine ~5% or so), pre-reacted in sodium hypochlorite aqueous solution, and then heated up to carry out Hoffman one-step reaction to obtain. Taking 4-aminopyridine as an example, the above method is used to synthesize, and the main steps are:
[0046] (1) Prepare an aqueous solution of 4-cyanopyridine (dissolve 171 g of 4-cyanopyridine in a reaction flask with 400 g of water to obtain an aqueous solution of 4-cyanopyridine), and cool the aqueous solution to below 0° C.;
[0047] (2) add catalyzer sodium tungstate, add-on is 2% of 4-cyanopyridine quality, promptly adds 3.4g catalyzer;
[0048] (3) then slowly add sodium hypochlorite solution dropwise (the mass ...
Embodiment 3
[0055] like figure 1 As shown, a kind of one-step method for synthesizing aminopyridine provided by the present invention, main steps comprise: under low temperature condition, with cyanopyridine as raw material, with sodium tungstate as catalyst (the addition of catalyst is raw material cyanopyridine quality About 2% to 5% of the sodium hypochlorite solution), pre-reacted in an aqueous solution of sodium hypochlorite, and then heated up to perform a Hoffmann one-step reaction to obtain. Taking 4-aminopyridine as an example, the above method is used to synthesize, and the main steps are:
[0056] (1) Prepare an aqueous solution of 4-cyanopyridine (in a reaction flask, dissolve 100 g of 4-cyanopyridine with 300 g of water to obtain an aqueous solution of 4-cyanopyridine), and cool the aqueous solution to below 0° C.;
[0057] (2) add catalyzer sodium tungstate, add-on is 3% of 4-cyanopyridine quality, promptly adds 3g catalyzer;
[0058] (3) then slowly add sodium hypochlorit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com