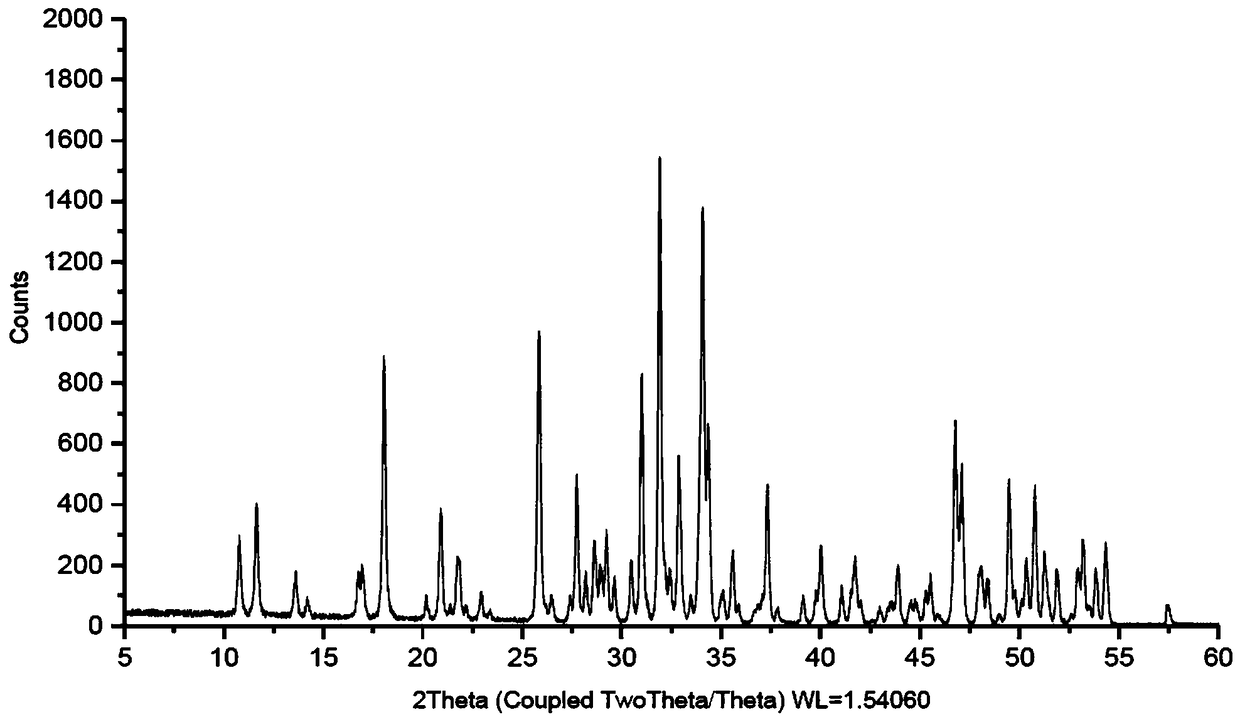
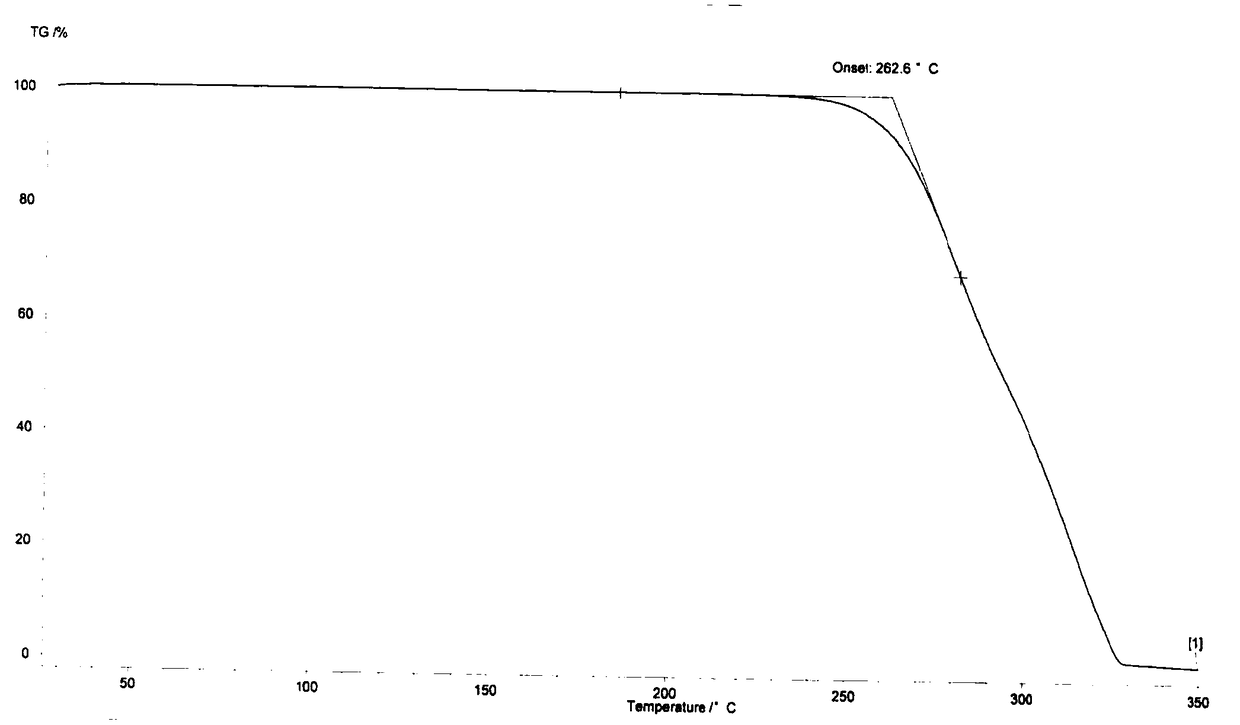
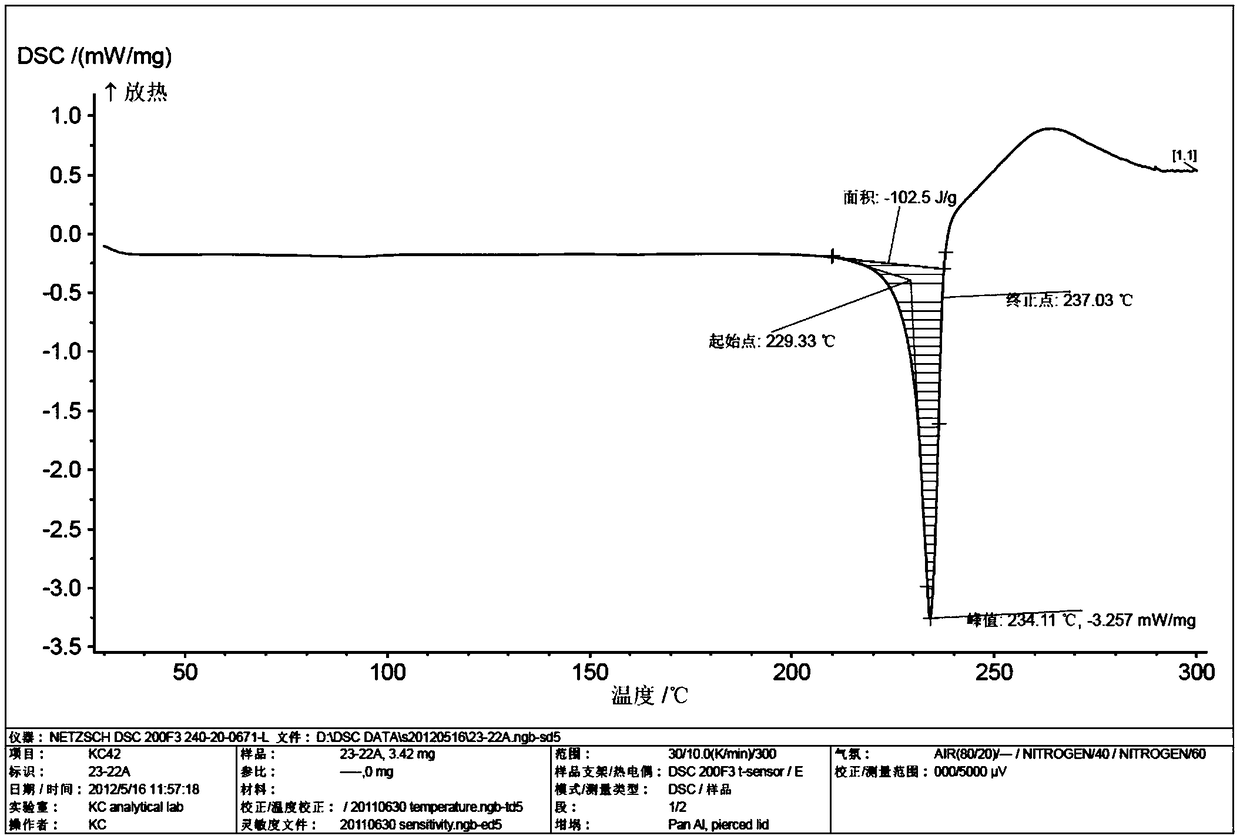
New crystal form of telaprevir and preparation method thereof
A telaprevir and crystal form technology, applied in the field of medicinal chemistry, can solve problems such as high cost investment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The present invention also provides a method for preparing the new crystal form of telapivir, the specific steps are:
[0025] The method for preparing the new crystal form of telaprevir is characterized in that it comprises the following steps:
[0026] Step (1) Take telaprevir, add a solvent, and heat to 70°C to dissolve;
[0027] Step (2) Bring the solution obtained in step (1) to 40-60°C, and add water dropwise;
[0028] Step (3) heat preservation and stirring until crystallization is complete, suction filtration, washing with water, and drying to obtain telaprevir.
[0029] Preferably, the solvent in step (1) is selected from methanol, ethanol, isopropanol, n-heptane, ethylene glycol dimethyl ether, a mixed solvent of water and methanol, a mixed solvent of water and ethanol, water and isopropyl A mixed solvent of alcohol, water and ethylene glycol dimethyl ether; the mass-volume ratio of the telaprevir to the solvent is 1g:10ml~1g:50ml; the step (2) the telaprevir and water...
specific Embodiment approach
[0033] The following examples are used to describe the technical solutions of the present invention in detail, which will help to have a better understanding of the advantages and effects of the technical solutions of the present invention. The examples do not limit the protection scope of the present invention. The protection scope of the present invention is determined by the claims. To decide.
Embodiment
[0035] The preparation method of crude telaprevir is as follows:
[0036] Step 1: Preparation of 2-((S)-2-((S)-2-amino-2-cyclohexylacetamido)-3,3-dimethylbutyryl)octahydrocyclopenta[c] Pyrrole-l-carboxylic acid (lS, 3aR, 6aS)-tert-butyl ester
[0037] A 3L three-neck round bottom flask equipped with a top stirrer, thermocouple, addition funnel, nitrogen outlet, and ice / water bath was charged with HOBt·H 2 O (51.74g, 1.05 molar equivalent), EDC·HCl (64.8g; 1.05 molar equivalent), then DMF (200ml) was charged, and stirring was started. The slurry was cooled to 0-5°C, and then a solution of 2-aminocyclohexanoacetic acid (98.45g; 1.05 molar equivalent) in DMF (172.4g; 182.9ml) was added and charged into the addition funnel. Add it to the batch for about 30 minutes, maintaining the temperature at 0-5°C. Once the addition is complete, the reaction mixture is stirred at 0-5°C for 2 hours. The 2-((S)-2-amino-3,3-dimethylbutyryl)-octahydrocyclopenta[c]pyrrole-1-carboxylic acid (lS, 3aR, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com