Ruthenium-doped ferronickel alloy catalyst for electrolysis water hydrogen production energy and preparation method
An iron-nickel alloy and electrolyzed water technology, which is applied in the electrolysis process, electrolysis components, metal processing equipment, etc., can solve the problems of poor hydrogen evolution performance, slow hydrogen evolution kinetics, and affecting the hydrogen production efficiency of electric price water, so as to improve the hydrogen evolution ability, Effects of lowering spontaneous electrolysis barrier and increasing reactive sites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Add nickel source, iron source, ruthenium source and chitosan into deionized water to prepare a sol; the nickel source is nickel chloride. The iron source was ferric chloride. The ruthenium source is ruthenium nitrate. In the sol, 10 parts by weight of nickel source, 20 parts by weight of iron source, 6 parts by weight of ruthenium source, 4 parts by weight of auxiliary agent, and 60 parts by weight of deionized water.
[0033] (2) The polyaniline-coated nickel foam powder was first immersed in the sol prepared in step (1), and then left to age until the system reached equilibrium; the time to stand and age was 5 hours.
[0034] (3) Filtration and drying, followed by high-temperature calcination in a reducing atmosphere to remove polyaniline, and obtain a ruthenium-doped iron-nickel alloy catalyst with a core-shell structure. The temperature of the high-temperature calcination is 700° C., and the time is 2 hours.
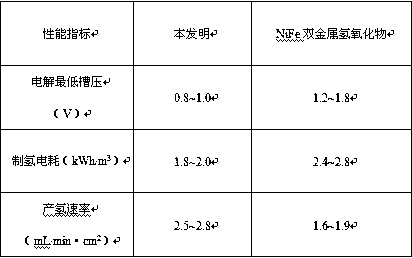
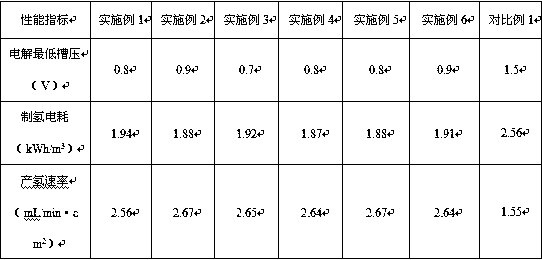
[0035] For the alloy catalyst prepared in Exampl...
Embodiment 2
[0037] (1) Add nickel source, iron source, ruthenium source and gelatin into deionized water to prepare a sol; the nickel source is nickel sulfate. The iron source was ferric sulfate. The ruthenium source is ruthenium trichloride. In the sol, 12 parts by weight of nickel source, 25 parts by weight of iron source, 6 parts by weight of ruthenium source, 2 parts by weight of auxiliary agent, and 55 parts by weight of deionized water.
[0038] (2) First immerse the polyaniline-coated nickel foam powder in the sol prepared in step (1), and then stand and age until the system reaches equilibrium; the time for standing and aging is 6 hours.
[0039] (3) Filtration and drying, followed by high-temperature calcination in a reducing atmosphere to remove polyaniline, and obtain a ruthenium-doped iron-nickel alloy catalyst with a core-shell structure. The temperature of high-temperature calcination is 500° C., and the time is 3 hours.
[0040] For the alloy catalyst prepared in Example...
Embodiment 3
[0042] (1) Add nickel source, iron source, ruthenium source and chitosan into deionized water to prepare a sol; the nickel source is nickel nitrate. The iron source was ferric nitrate. The ruthenium source is ruthenium nitrate. In the sol, 10 parts by weight of nickel source, 22 parts by weight of iron source, 6 parts by weight of ruthenium source, 2 parts by weight of auxiliary agent, and 60 parts by weight of deionized water.
[0043] (2) First immerse the polyaniline-coated nickel foam powder in the sol prepared in step (1), and then stand and age until the system reaches equilibrium; the time for standing and aging is 4 hours.
[0044] (3) Filtration and drying, followed by high-temperature calcination in a reducing atmosphere to remove polyaniline, and obtain a ruthenium-doped iron-nickel alloy catalyst with a core-shell structure. The temperature of high-temperature calcination is 650° C., and the time is 2 hours.
[0045] For the alloy catalyst prepared in Example 3,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com