Anhydrous CO2 (carbon dioxide) phase change absorbent with low energy consumption and regeneration method and application thereof
An absorbent, low-energy-consumption technology, applied in the fields of recovery, carbon dioxide capture, and separation, can solve the problems of high cost of ionic liquids, complex regeneration process, and complex separation process, and is conducive to industrialization, reducing phase separation processes, and reducing The effect of energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
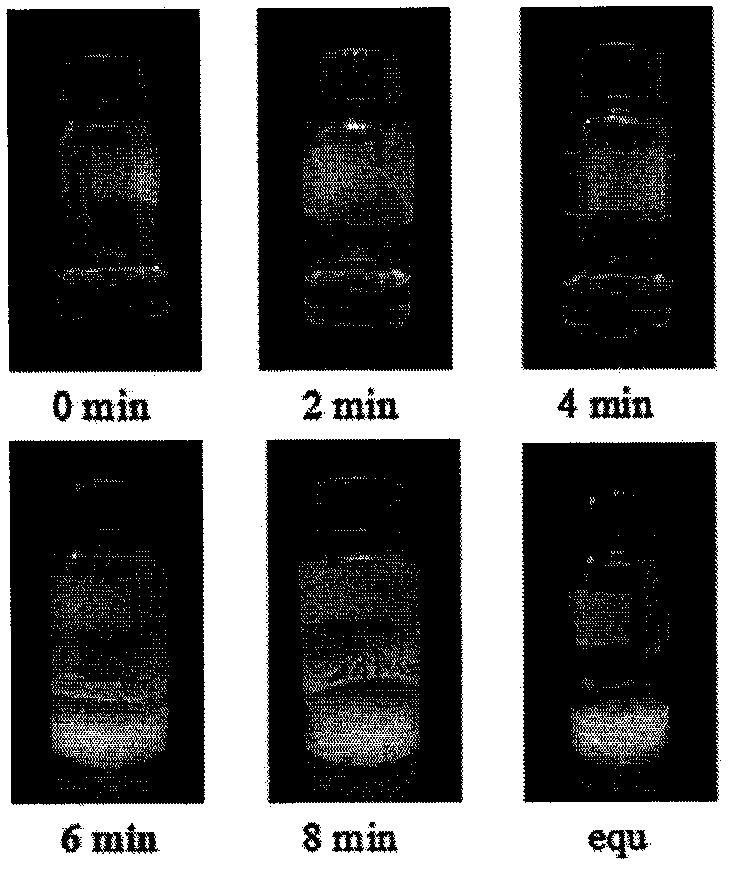
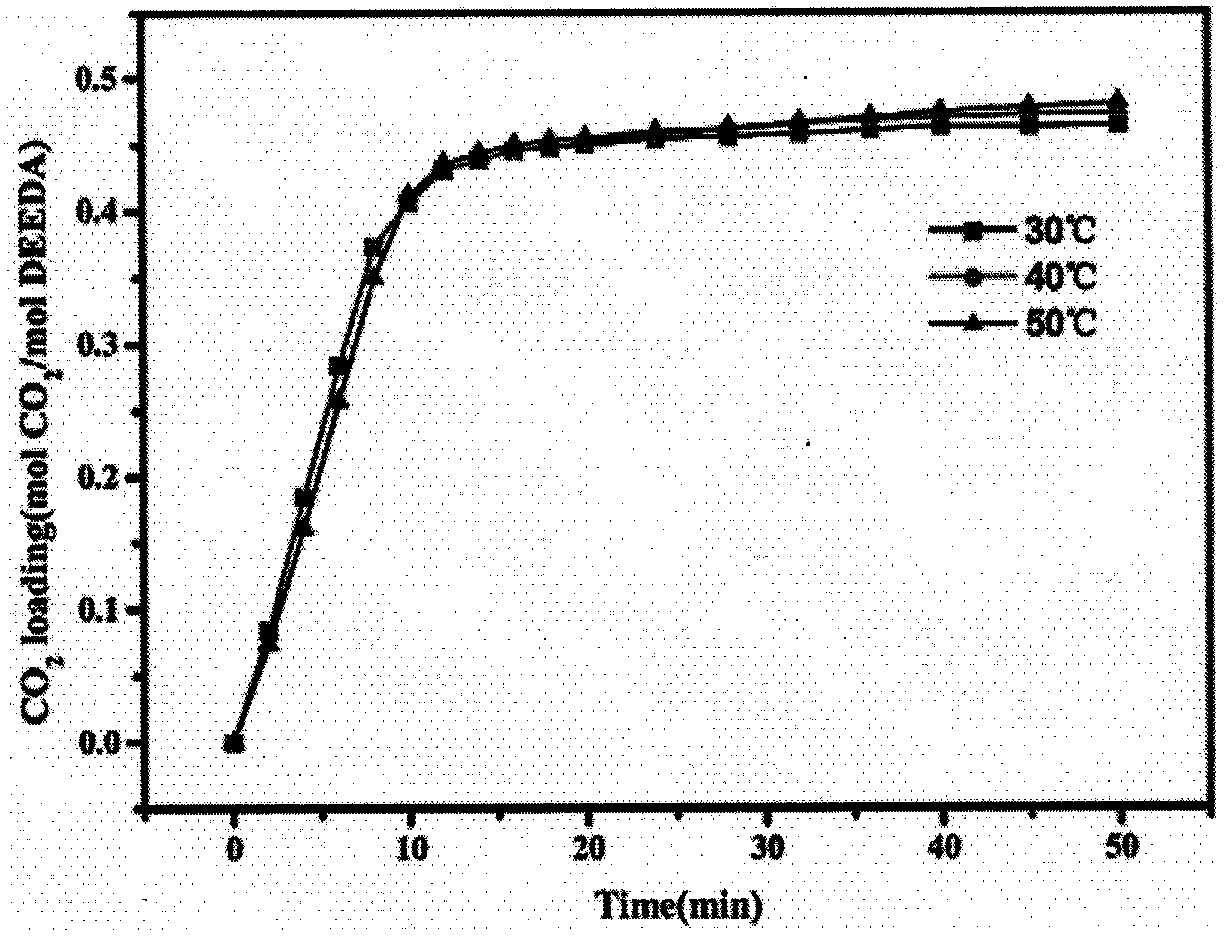
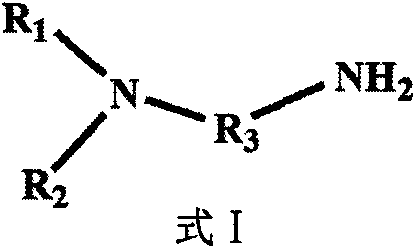
[0045] Absorbent of the present invention adopts primary amine (NH 2 -) and tertiary amine (-N-) a single diamine compound N, N'-dimethylethylenediamine, the concentration is 100%, does not contain any other organic solvents, water and ionic liquid, wherein the tertiary amine nitrogen There are two alkyl branch chains linked on the atom, which constitute a certain degree of hydrophobicity. Its molecular structure formula is Absorbent N of the present invention, N'-dimethylethylenediamine absorbs CO 2 when CO 2 The flow rate is 25ml / min, and the absorption saturation is reached in 10min, and the CO of the absorbent 2 Loading capacity is 0.465mol CO 2 / mol amine, the mechanism of the phase change reaction of the absorbent is: A low energy consumption anhydrous CO of the present invention 2 The regeneration method of phase-change absorbent, comprises the following steps:
[0046] 1) Sealed sampling: take 2.80g absorbent to absorb CO 2 The final carbamate solid is sealed ...
Embodiment 2
[0054] Absorbent of the present invention adopts primary amine (NH 2 -) and tertiary amine (-N-) a single diamine compound N, N'-diethylethylenediamine, the concentration is 100%, does not contain any other organic solvents, water and ionic liquid, wherein the tertiary amine nitrogen There are two alkyl branch chains linked on the atom, which constitute a certain degree of hydrophobicity. Its molecular structure formula is Absorbent N of the present invention, N'-diethylethylenediamine absorbs CO 2 when CO 2 The flow rate is 30ml / min, and the absorption saturation is reached in 12min, and the CO of the absorbent 2 Loading capacity is 0.464mol CO 2 / mol amine, the phase change reaction mechanism of the adsorbent is: A low energy consumption anhydrous CO of the present invention 2 The regeneration method of phase-change absorbent, comprises the following steps:
[0055] 1) Sealed sampling: take 3.10g absorbent to absorb CO 2 The final carbamate solid is sealed in a 20ml...
Embodiment 3
[0063] Absorbent of the present invention adopts primary amine (NH 2 -) and tertiary amine (-N-), the single diamine compound N, N'-diisopropylethylenediamine, the concentration is 100%, does not contain any other organic solvents, water and ionic liquid, wherein the tertiary amine There are two alkyl branch chains linked to the nitrogen atom, which constitute a certain degree of hydrophobicity. Its molecular structure formula Absorbent N of the present invention, N'-diisopropylethylenediamine absorbs CO 2 when CO 2 The flow rate is 35ml / min, the absorption saturation is reached in 9min, and the CO of the absorbent 2 The loading capacity is 0.413mol CO2 / mol amine, and the phase change reaction mechanism of the adsorbent is as follows: A low energy consumption anhydrous CO of the present invention 2 The regeneration method of phase-change absorbent, comprises the following steps:
[0064] 1) Sealed sampling: take 3.30g absorbent to absorb CO 2 The final carbamate solid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com