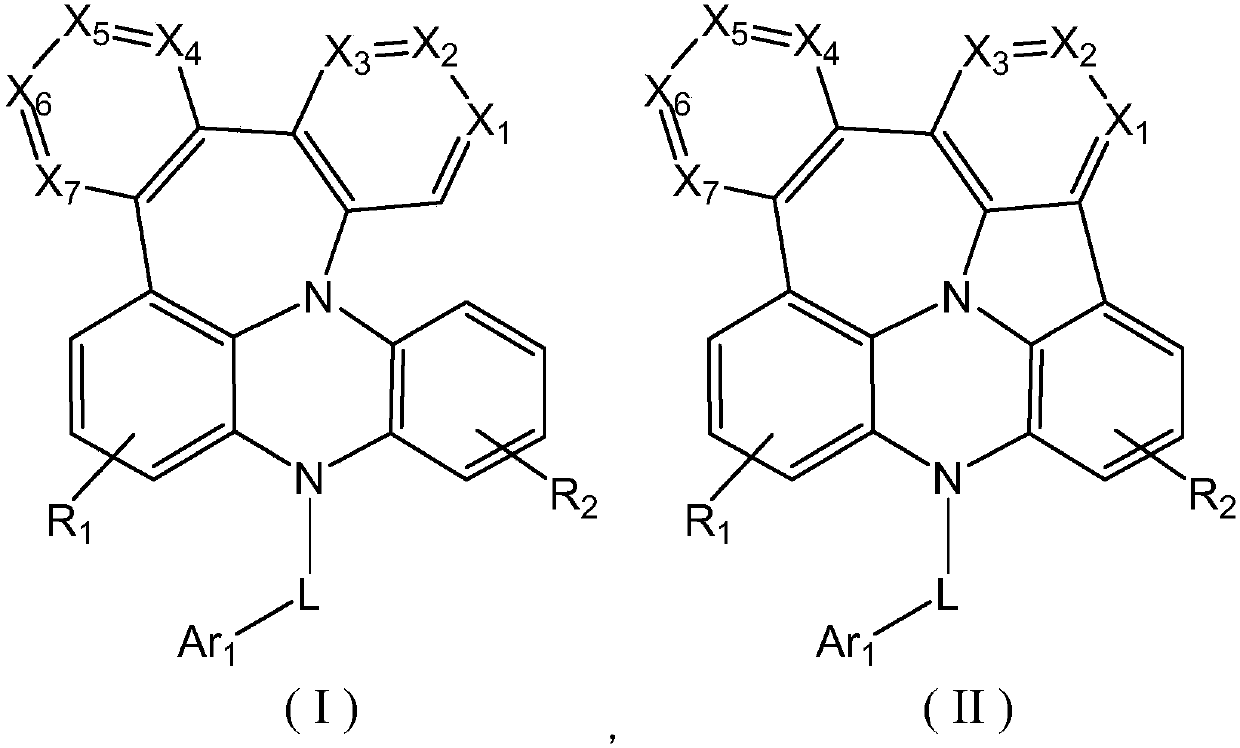
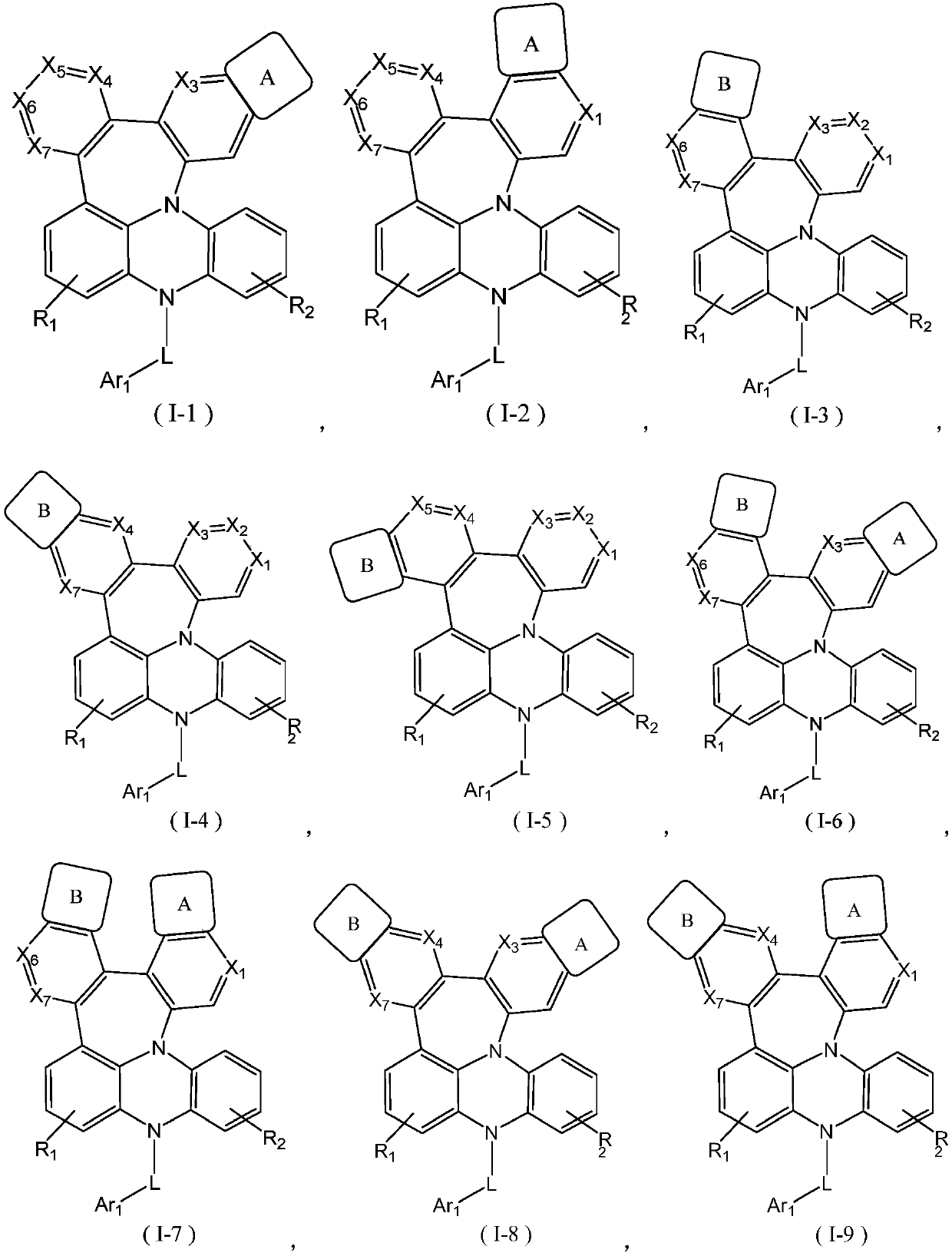
Fused ring compound as well as preparation method and purpose thereof
A compound and fused ring technology, applied in the field of fused ring compounds and their preparation, can solve the problems of unsatisfactory light-emitting region, inefficient energy transfer of host materials, and easy crystallization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] This embodiment provides a condensed ring compound, which has the structure shown in the following formula D-1:
[0072]
[0073] The synthetic route of the condensed ring compound shown in formula D-1 is as follows:
[0074]
[0075] The preparation method of the condensed ring compound shown in formula D-1 specifically comprises the following steps:
[0076] (1) Synthesis of Intermediate 1-1
[0077] Under nitrogen protection, weigh 10g of compound (33mmol) shown in formula (A-1), 6.7g 1-bromo-2-nitrobenzene (33mmol) (compound shown in formula (B-1)), 0.2g palladium acetate (1.0mmol), 0.66g tri-tert-butylphosphine (3.5mmol), 9.3g sodium tert-butoxide, 1000mL toluene, reacted at 110°C for 12 hours, cooled to room temperature, extracted with chloroform, rotary evaporated to remove the solvent, and passed through a silica gel column to obtain 11.0 g solid intermediate 1-1 (yield 85%);
[0078] (2) Synthesis of Intermediate 2-1
[0079] Under the protection of n...
Embodiment 2
[0084] This embodiment provides a condensed ring compound, which has the structure shown in the following formula D-2:
[0085]
[0086] The synthetic route of the condensed ring compound shown in formula D-2 is as follows:
[0087]
[0088] The preparation method of the condensed ring compound shown in formula D-2 specifically comprises the following steps:
[0089] (1) Using the compound shown in formula (A-2) and the compound shown in formula (B-1) as starting materials, according to the synthetic method in Example 1, synthesize intermediate 2-2;
[0090] (2) Synthesis of condensed ring compound D-2
[0091] Under nitrogen protection, add 3.8g intermediate 2-2 (10mmol), 3.2g compound (12mmol), 3.4g cesium carbonate (10mmol), 0.6g 4-dimethylaminopyridine (5mmol), dimethyl sulfoxide 40mL, 100 ℃ reaction 3 hours, after cooling to room temperature, toluene extraction, rotary evaporation removes solvent, Pass through a silica gel column to obtain 5.0 g of solid condens...
Embodiment 3
[0094] This embodiment provides a condensed ring compound, which has the structure shown in the following formula D-8:
[0095]
[0096] The synthetic route of the condensed ring compound shown in formula D-8 is as follows:
[0097]
[0098] The preparation method of the condensed ring compound shown in formula D-8 specifically comprises the following steps:
[0099] (1) Synthesize intermediate 2-2 with the synthetic method in Example 2;
[0100] (2) Under nitrogen protection, add 3.8g intermediate 2-2 (10mmol), 0.09g palladium acetate (0.4mmol), 0.29g tri-tert-butylphosphine (1.42mmol), 4.5g (12mmol), 4.2g sodium tert-butoxide, 250mL toluene, reacted at 110°C for 12 hours, cooled to room temperature, extracted with chloroform, and removed the solvent by rotary evaporation, and passed through a silica gel column to obtain 5.7g solid condensed ring compound D-8 (yield: 82 %).
[0101] Elemental analysis: (C49H31N5) Theoretical value: C, 85.32; H, 4.53; N, 10.15; Found...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com