Nanometer photosensitizer for photodynamic antisepsis, preparation method and application thereof
A photosensitizer and nanotechnology, applied in the direction of antibacterial drugs, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of low solubility, instability, easy aggregation, etc., and achieve improved hydrophobicity , Improve the effect of stability and biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
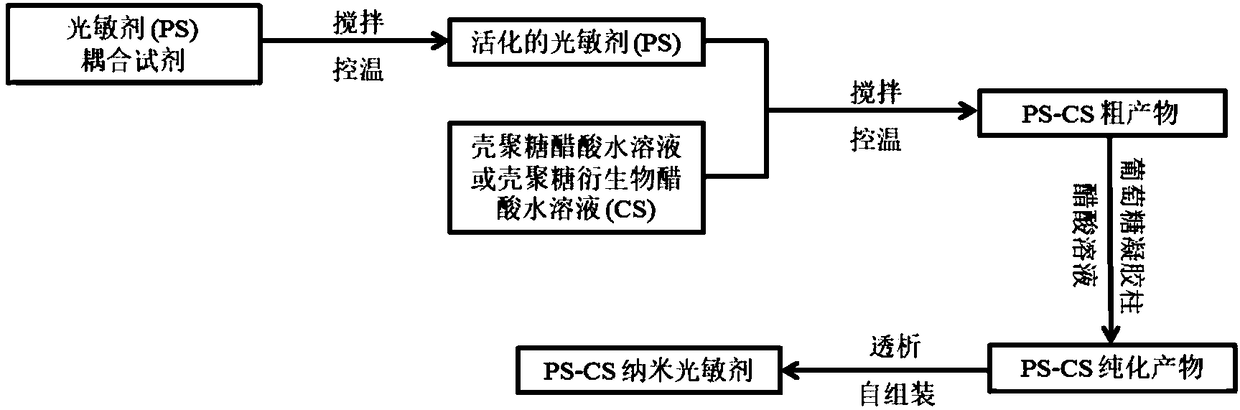
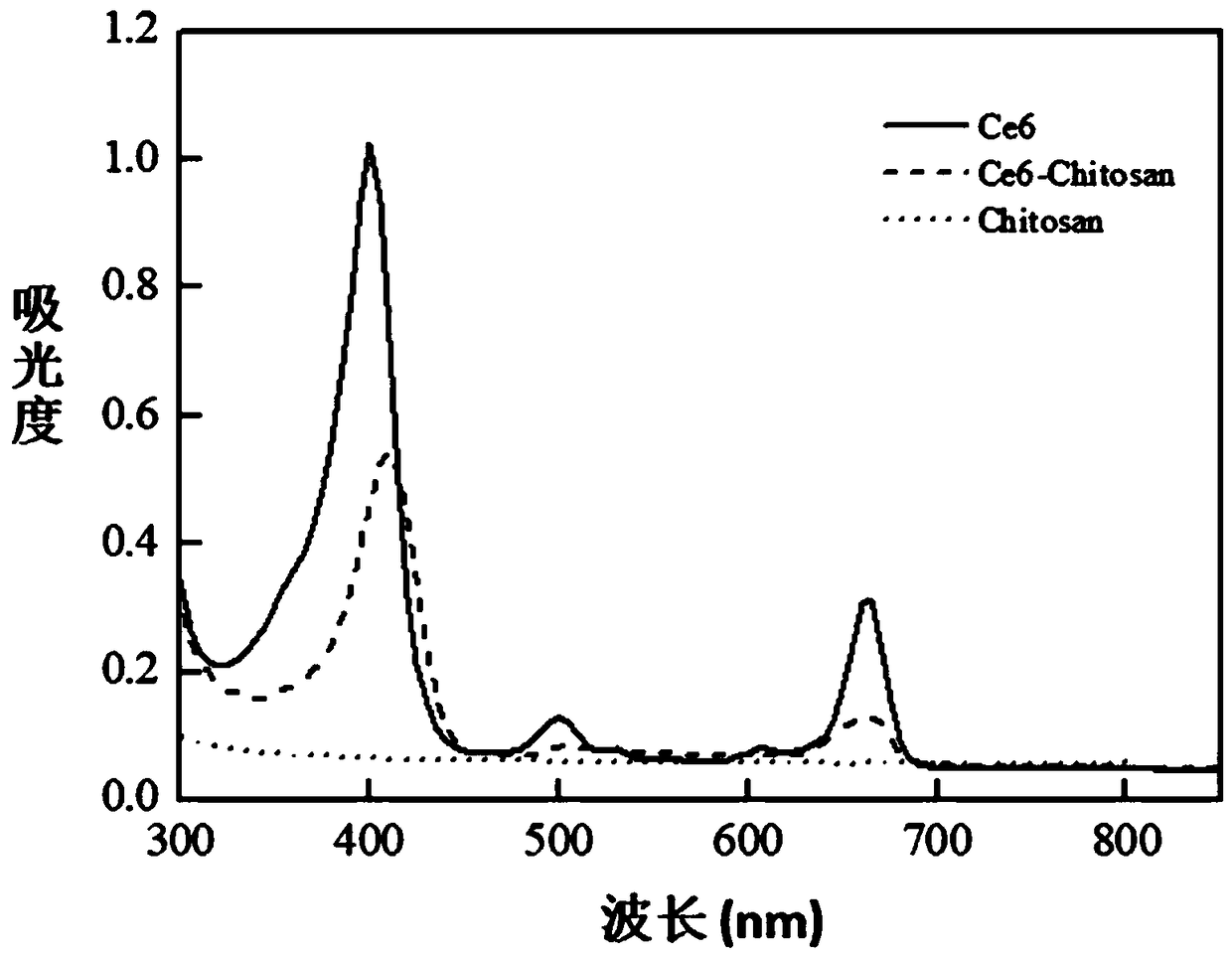
[0041] S1: Weigh N,N'-Dicyclohexylcarbodiimide (DCC) 6.9mg, N-Hydroxysuccinimide (NHS) 4.0mg and Chlorin e6 (Ce6) 10mg and dissolve in 10mL. Dimethyl sulfoxide (DMSO), stirred for 0.5 h at 25°C and protected from light to obtain an activated chlorin e6 solution;
[0042] S2: After the activated chlorin e6 solution is filtered with a filter to remove insoluble by-products, it is slowly added dropwise to a 0.5% chitosan acetic acid aqueous solution (100 mL), and protected from light at 25°C Stir the reaction for 12 hours to obtain the crude product of chlorin e6 grafted chitosan (Ce6-Chitosan);
[0043] S3: Take the crude product solution of chlorin e6 grafted chitosan (Ce6-Chitosan) and add it dropwise to the top of the dextran gel column. After it enters the gel, it is eluted with 0.5% acetic acid aqueous solution. The purified product of chlorin e6 grafted with chitosan (Ce6-Chitosan) was obtained.
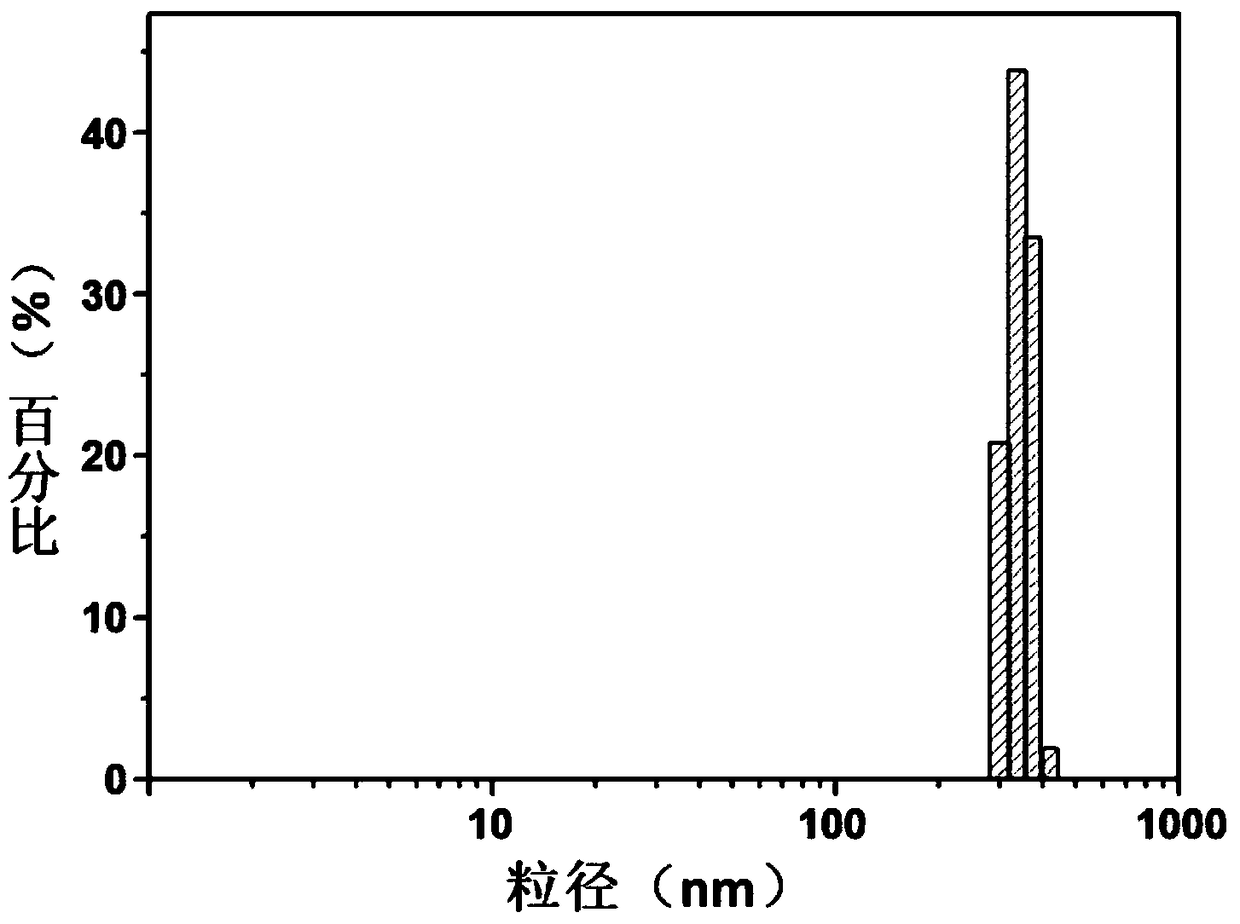
[0044] S4: The purified product of chlorin e6 grafted with chitosan (Ce6-Chitosan...
Embodiment 2
[0046] S1: Weigh triethylamine (Et 3 N) 8.4mg, O-benzotriazole-tetramethylurea hexafluorophosphate (HBTU) 31.6mg and chlorin e6 (Ce6) 10mg mixed and dissolved in 10mL dimethylformamide (DMF), After stirring for 12 hours at 20°C in the dark to obtain an activated chlorin e6 solution;
[0047] S2: After the activated chlorin e6 solution is filtered with a membrane to remove insoluble by-products, it is slowly added dropwise to 3.2% N-(2-hydroxypropyltrimethyl) chitosan acetic acid aqueous solution (100mL), the reaction was stirred for 24h in the dark at 20°C to obtain the crude product of chlorin e6 grafted with N-(2-hydroxypropyltrimethyl) chitosan;
[0048] S3: Take the crude product solution of chlorin e6 grafted N-(2-hydroxypropyltrimethyl) chitosan and drop it on the top of the dextran gel column. After entering the gel, use the mass fraction as 1% acetic acid aqueous solution was eluted and purified to obtain the purified product of chlorin e6 grafted with N-(2-hydroxypropyltr...
Embodiment 3
[0051] S1: Weigh respectively 17.3mg of N,N-diisopropylethylamine (DIPEA), O-benzotriazole-N,N,N',N'-tetramethylurea tetrafluoroborate (TBTU ) 21.4 mg and 5 mg of Chlorin e6 (Ce6) were mixed and dissolved in 10 mL of dimethylformamide (DMF). After stirring for 6 hours at 30°C and protected from light, an activated Chlorin e6 solution was obtained;
[0052] S2: After the activated chlorin e6 solution is filtered with a filter membrane to remove insoluble by-products, it is slowly added dropwise to 3.2% chitosan α-aminophenoxypyrimidine phosphonate ethyl acetate aqueous solution ( 50mL), react for 48h in the dark at 30°C to obtain the crude product of chlorin e6 grafted chitosan ethyl α-aminophenoxypyrimidine phosphonate;
[0053] S3: Take the crude product solution of chlorin e6 grafted chitosan α-aminophenoxypyrimidine phosphonate and drop it onto the top of the dextran gel column. After entering the gel, use a mass fraction of 2 % Acetic acid aqueous solution was eluted and purif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com