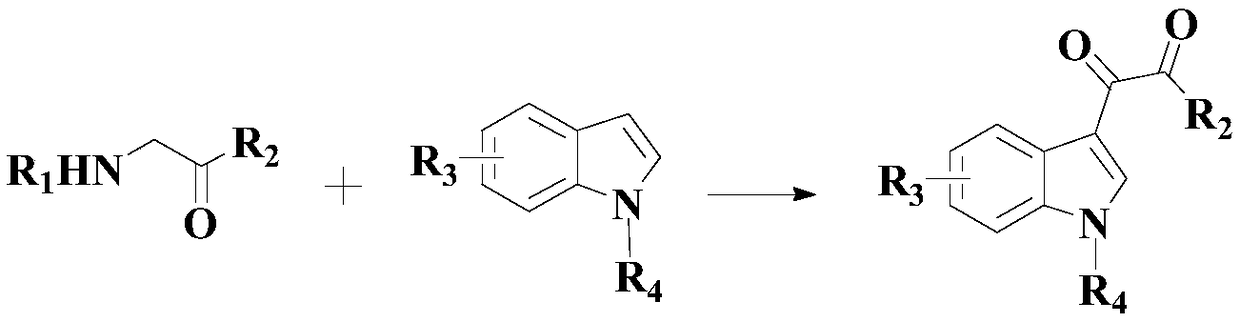
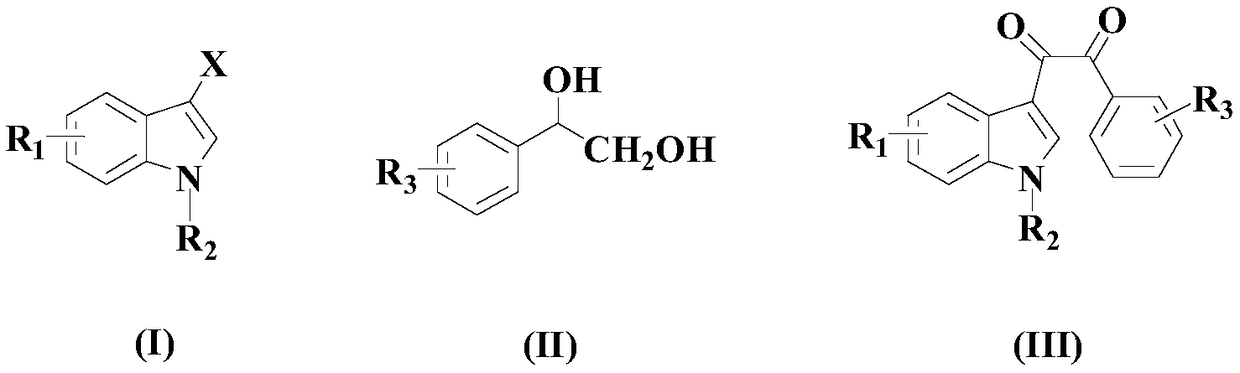
Synthetic method of diaryl-substituted dicarbonyl compound
A technology of a dicarbonyl compound and a synthesis method, which is applied in the field of organic chemical synthesis, can solve the problems of low product yield and the like, and achieve the effect of good application prospect and industrial production potential.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
[0037] In an appropriate amount of organic solvent at room temperature (a mixture of dimethyl sulfoxide (DMSO) and acetonitrile at a volume ratio of 3:1), add 100 mmol of the above formula (I) compound, 120 mmol of the above formula (II) compound, 20 mmol Two-component composite catalyst (composed of 10mmol 1,3-bis(diphenylphosphinopropane) nickel dichloride and 10mmol copper hexafluoroacetylacetonate), 200mmol oxidant 2-iodylbenzoic acid (IBX), 150mmol base dimethyl Aminopyridine (DMPA) and 14mmol auxiliary tetraphenylporphyrin, then heated to 70°C while stirring, and stirred and reacted at this temperature for 9 hours;
[0038] After the reaction is completed, quench the reaction with deionized water, filter, fully wash the filtrate with deionized water, then extract the organic phase with ethyl acetate for 2-3 times, combine the organic phases and dry them with anhydrous sodium sulfate, and finally distill under reduced pressure. The viscous residue obtained was...
Embodiment 2
[0041]
[0042] To an appropriate amount of organic solvent at room temperature (a mixture of dimethyl sulfoxide (DMSO) and acetonitrile at a volume ratio of 3:1), add 100 mmol of the above formula (I) compound, 180 mmol of the above formula (II) compound, 10 mmol Two-component composite catalyst (composed of 5mmol 1,3-bis(diphenylphosphinopropane) nickel dichloride and 5mmol copper hexafluoroacetylacetonate), 250mmol oxidant 2-iodylbenzoic acid (IBX), 100mmol alkali dimethyl Aminopyridine (DMPA) and 18mmol auxiliary tetraphenylporphyrin, then heated to 100°C while stirring, and stirred and reacted at this temperature for 6 hours;
[0043] After the reaction is completed, quench the reaction with deionized water, filter, fully wash the filtrate with deionized water, then extract the organic phase with ethyl acetate for 2-3 times, combine the organic phases and dry them with anhydrous sodium sulfate, and finally distill under reduced pressure. The viscous residue obtained wa...
Embodiment 3
[0046]
[0047] In an appropriate amount of organic solvent at room temperature (a mixture of dimethyl sulfoxide (DMSO) and acetonitrile at a volume ratio of 3:1), add 100 mmol of the above formula (I) compound, 150 mmol of the above formula (II) compound, 16 mmol Two-component composite catalyst (composed of 8mmol 1,3-bis(diphenylphosphinopropane) nickel dichloride and 8mmol copper hexafluoroacetylacetonate), 225mmol oxidant 2-iodylbenzoic acid (IBX), 125mmol alkali dimethyl Aminopyridine (DMPA) and 16mmol auxiliary tetraphenylporphyrin, then heated to 80°C while stirring, and stirred and reacted at this temperature for 8 hours;
[0048] After the reaction is completed, quench the reaction with deionized water, filter, fully wash the filtrate with deionized water, then extract the organic phase with ethyl acetate for 2-3 times, combine the organic phases and dry them with anhydrous sodium sulfate, and finally distill under reduced pressure. The viscous residue obtained was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com