Preparation method and applications of boron suboxide (B6O)
A boron powder and reaction technology, applied in the field of photocatalytic materials, can solve the problems of unfavorable large-scale application, poor stability and easy deactivation, etc., and achieve the effect of favorable catalytic reaction, high stability and good visible light catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of boron hexaoxide
[0030] (1) Place the amorphous boron powder in a tube furnace, and bake it at 1200°C for 600 minutes under an argon atmosphere to convert it into boron powder with a certain degree of crystallinity;
[0031] (2) Add 1 g of boron powder with crystallinity obtained in step (1) into 100 mL of 30 wt % hydrogen peroxide solution, then ultrasonically disperse with an ultrasonic instrument at 20° C. for 20 minutes, and then react at 60° C. for 60 minutes;
[0032] (3) Step (2) After the reaction in step (2), centrifuge and separate, wash three times with deionized water and ethanol respectively, and then dry the obtained powder in an oven at 60° C. for 6 hours to obtain boron hexaoxide.
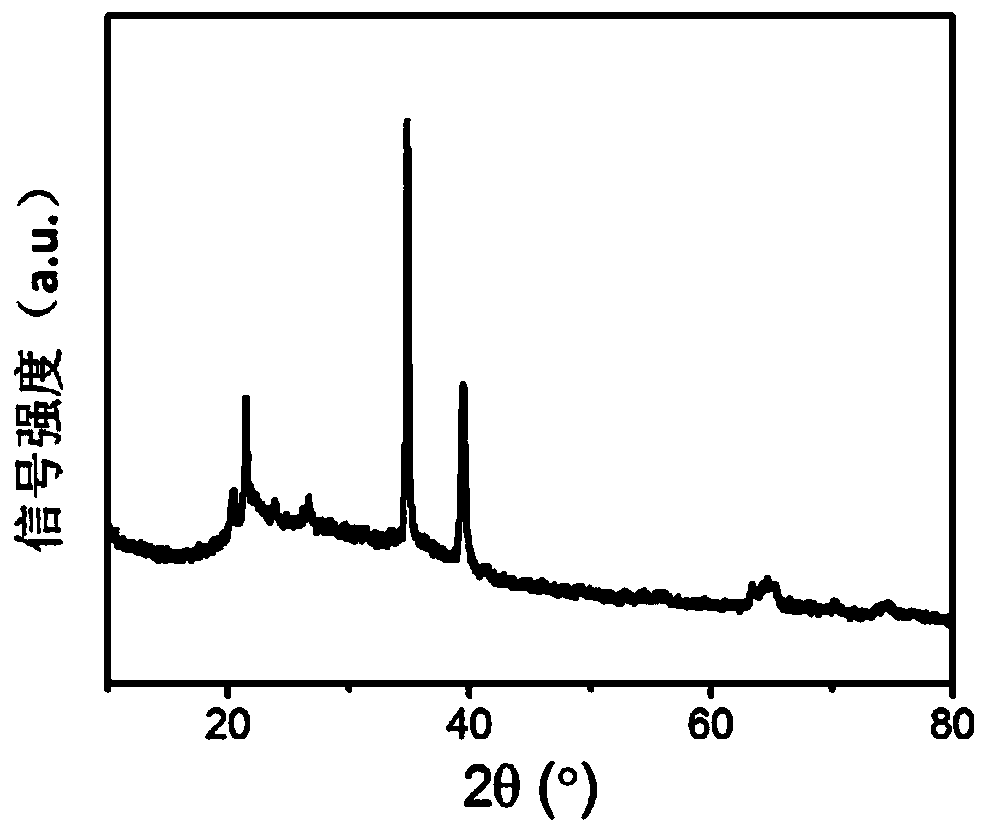
[0033] tested as figure 1 The X-ray diffraction pattern is shown, and the purity of boron hexaoxide is >98%.
Embodiment 2
[0035] Preparation of boron hexaoxide
[0036] (1) Place the amorphous boron powder in a tube furnace, and calcine it at 1000°C for 120 minutes under an argon atmosphere to convert it into boron powder with a certain degree of crystallinity;
[0037] (2) Slowly add 1 g of boron powder with crystallinity obtained in step (1) into 100 ml of 60 wt % concentrated nitric acid solution, and then react at 70° C. for 60 min;
[0038] (3) The solution after the reaction in step (2) was diluted and centrifuged, washed three times with deionized water and ethanol respectively, and then the obtained powder was dried in an oven at 80° C. for 6 hours to obtain boron hexaoxide.
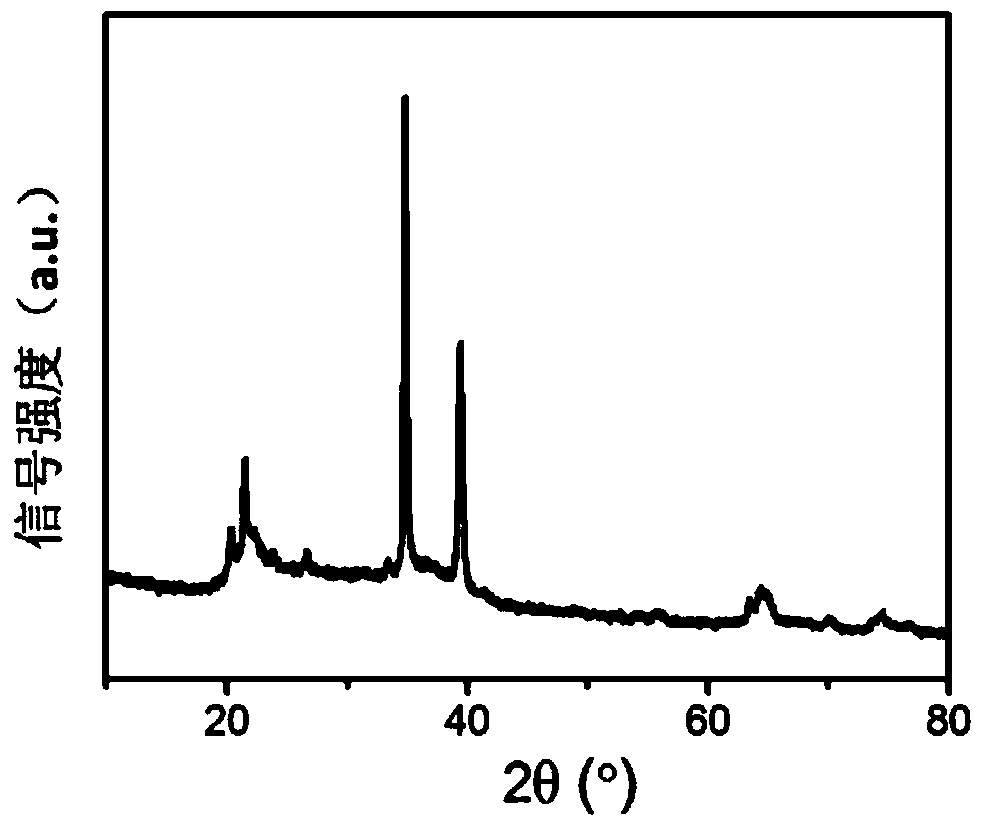
[0039] tested as figure 2 The X-ray diffraction pattern is shown, and the purity of boron hexaoxide is >98%.
Embodiment 3
[0041] Preparation of boron hexaoxide
[0042] (1) Raw material boron powder adopts boron powder with crystallinity. Add 1g of boron powder with crystallinity into 100mL of 30wt% hydrogen peroxide solution, then ultrasonically disperse with an ultrasonic instrument at 20°C for 10min, and then react at 100°C for 240min;
[0043] (2) The solution after the reaction in step (1) was diluted and then centrifuged, washed three times with deionized water and ethanol respectively, and then the obtained powder was oven-dried at 50° C. for 6 hours to obtain boron hexaoxide.
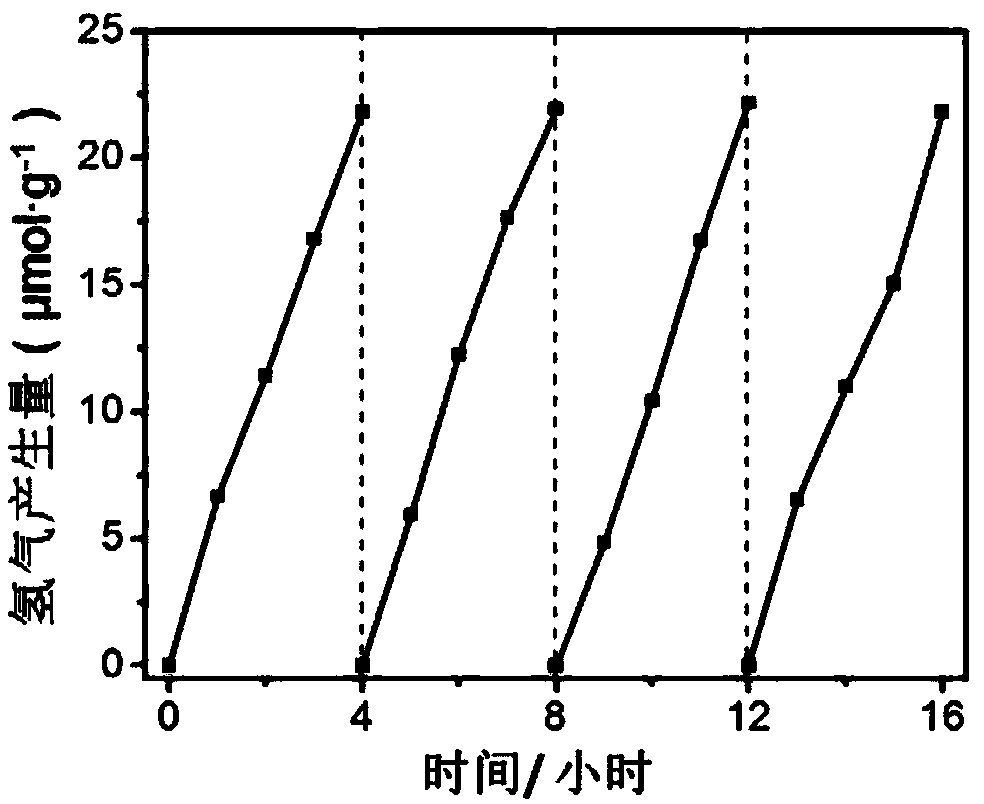
[0044] After testing, the purity of boron hexaoxide is >99%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com