A kind of preparation method of polyester containing carbon-carbon double bond
A technology of carbon double bond and polyester, which is applied in the field of preparation of carbon-carbon double bond polyester, can solve the problems of few modification points, low modification efficiency and difficult control, and achieve simple operation, controllable reaction and easy preparation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1: synthesis contains the polycaprolactone of phenylselenyl group
[0055] Synthetic polycaprolactone containing phenylselenyl group, comprises the following steps:
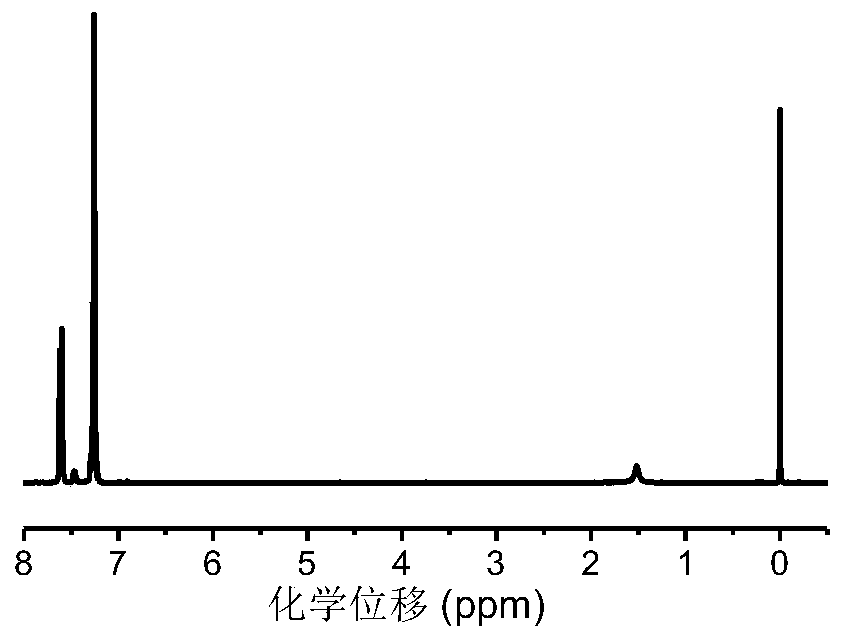
[0056] Preparation of diphenyl diselenide (DPDS): install a thermometer, a constant pressure dropping funnel on a 250mL three-necked flask, and a spherical condenser with an anhydrous calcium chloride drying tube on the top. Weigh 2.4g (0.10mol) of magnesium chips and a catalytic amount of iodine into a three-necked flask, weigh 15.7g (0.10mol) of bromobenzene and 58mL of anhydrous ether into a constant pressure dropping funnel, and slowly drop them into the reactor. The reaction was exothermic, cooled in an ice-water bath to keep the reaction system slightly boiling, and continued to react at 35°C (boiling) for about 1 hour after the dropwise addition, and the magnesium chips basically reacted completely. Then 7.9g (0.10mol) of selenium powder was added in batches, and the reaction was refluxe...
Embodiment 2
[0061] Example 2: Research on PhSeCL Aggregation Behavior
[0062] In the glove box, add PhSeCL (0.5g, 1.8mmol), EG (6.2μL, 0.11mmol), Sn(Oct) in a 10mL ampoule 2 (36 μL, 0.11 mmol) and 2 mL of anhydrous toluene. Then the solution was divided into 5 parts in 2mL ampoules, and the tubes were sealed. The ampoules were then placed in a preheated 100°C heating plate and stirred for the set time. The solution was diluted with THF, precipitated in anhydrous ether, centrifuged to obtain the product, and dried in a vacuum oven at 25°C to constant weight. pass 1 H NMR was used to calculate the monomer conversion rate, and the molecular weight and molecular weight distribution of the polymer were measured by GPC.
[0063] attached Figure 4 The displayed GPC elution curve is unimodal and symmetrical. And attached Figure 5 The middle kinetics presents a pseudo first-order linear relationship, the molecular weight of the polymer increases linearly with the increase of the monomer ...
Embodiment 3
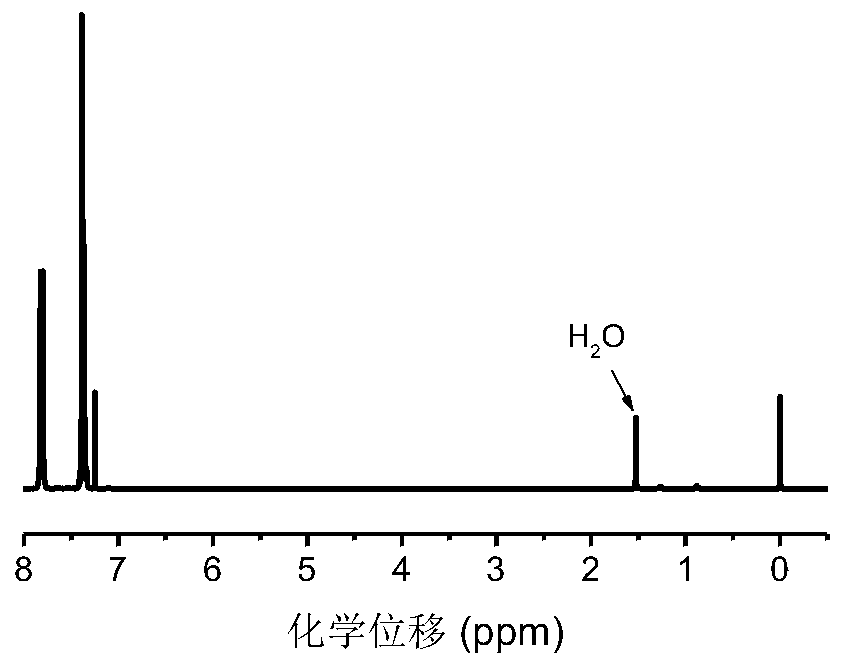
[0064] Example 3: Oxidation Reaction
[0065] According to the molar ratio of phenylselenyl group in the polymer: m-chloroperoxybenzoic acid=1:10, add the polymer, a certain amount of THF solvent, and m-chloroperoxybenzoic acid in a clean and dry 10mL ampoule, and stir at room temperature for 30 minutes Afterwards, ether was precipitated, centrifuged, and dried.
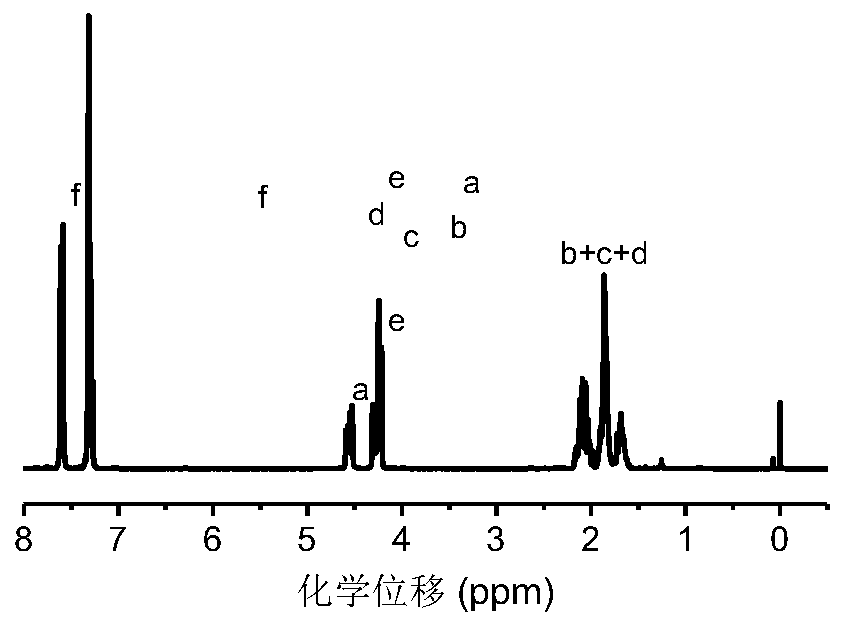
[0066] attached Figure 7 It can be seen from the GPC elution curve that the polymer peak shifts to a higher retention time. Due to the elimination of the phenyl selenide group, the molecular weight of the polymer changes from 9200g mol -1 Reduced to 7600g mol -1 , and the molecular weight distribution of the polymer is still very narrow It shows that the oxidation reaction has indeed occurred. attached Figure 8 After NMR oxidation, the resonance signal at chemical shift 7.60-7.15ppm disappeared, and the resonance signal at 3.63-3.46ppm on the methine group connected to selenium also disappeared, and at the sa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com