Test strip card for quantitative detection of classical swine fever virus antibody with double antigen sandwich and double detection line
A double-antigen sandwich, swine fever virus technology, applied in measurement devices, biological tests, material inspection products, etc., can solve the problems of insignificant difference in detection color and low sensitivity, and achieve improved sensitivity, sufficient response, and increased response time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
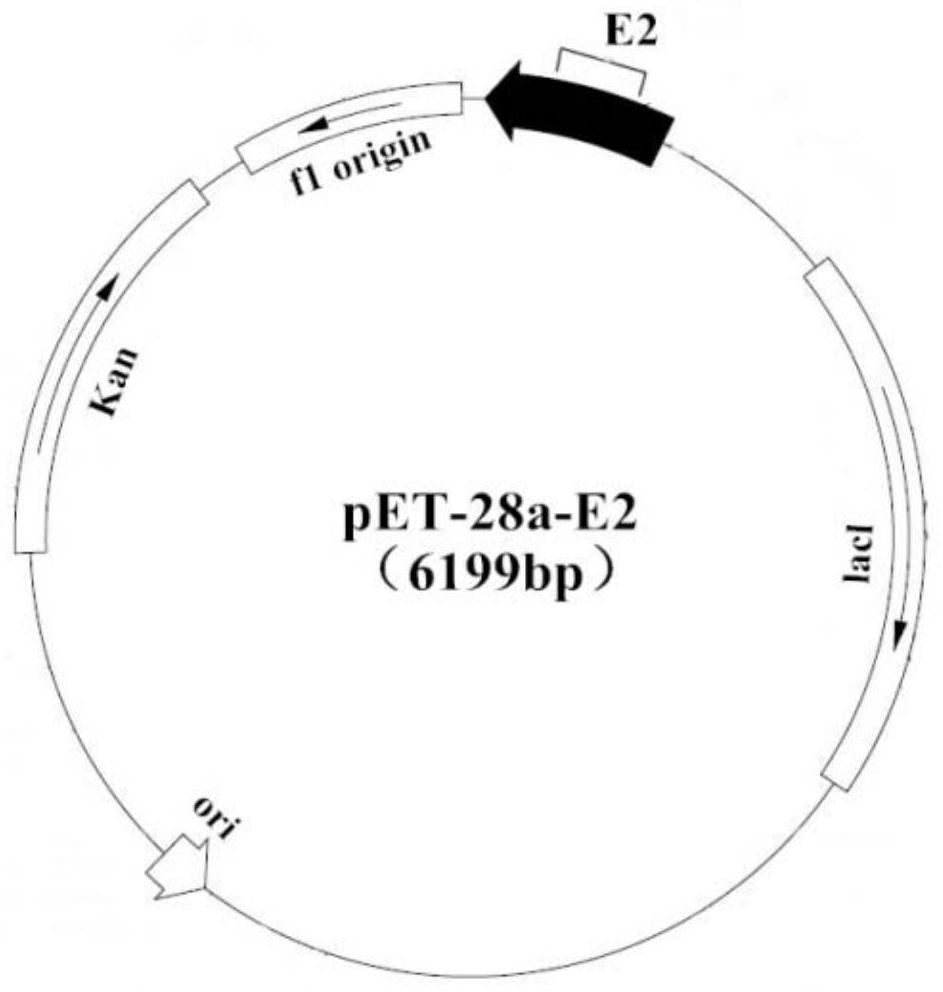
[0046] The preparation of embodiment 1 swine fever virus E2 and E2a recombinant antigen
[0047] This example provides a preparation method of recombinant antigens of classical swine fever virus E2 and E2a as follows: use IPTG to induce recombinant strains producing E2 and E2a proteins respectively, and the expression products are purified to obtain recombinant antigens of classical swine fever virus E2 and classical swine fever virus E2a respectively recombinant antigen.
[0048] (1) The preparation method of CSFV E2 recombinant antigen is as follows:
[0049] The classical swine fever virus E2 antigen protein genome sequence was searched in the NCBI database, and GenBank: FJ598611.1 (Classical swine fever virus strain C-strain-ZJ E2glycoprotein gene) was selected as the target gene. The specific gene sequence is shown in SEQ ID NO: 1.
[0050] Use the Primer premier5 software to design the PCR primers for amplifying the E2 gene, and the sequences of the primers are as follo...
Embodiment 2
[0068] The assembly of embodiment 2 swine fever virus antibody quantitative detection test paper card
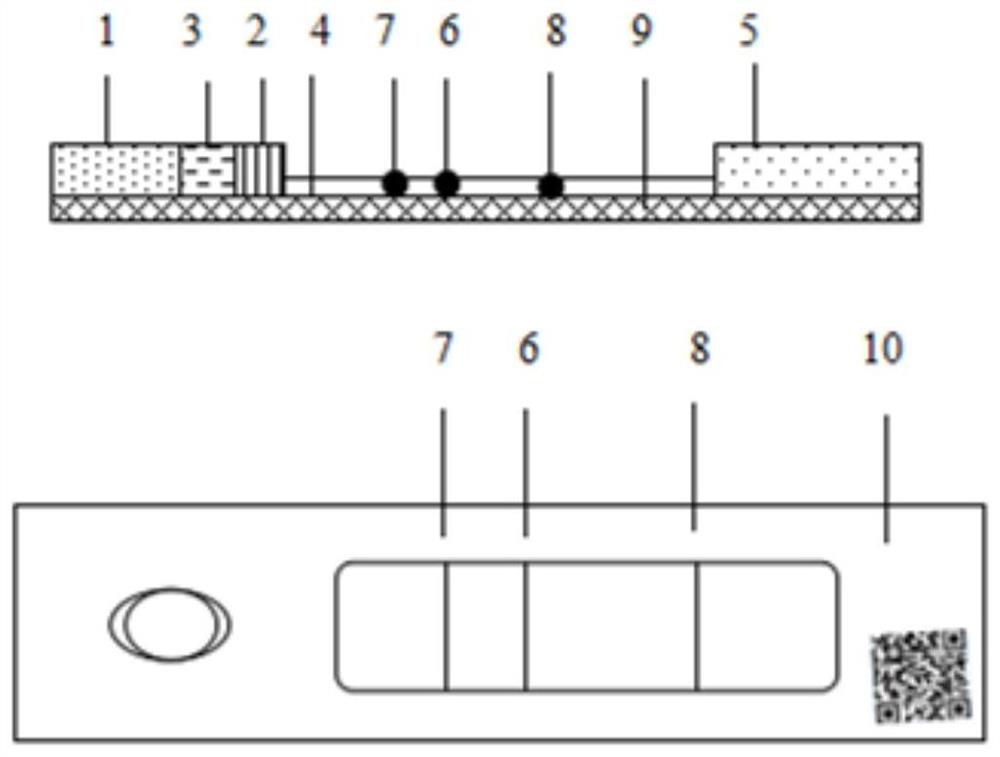
[0069] The embodiment of the present invention provides a preparation method of a test paper card for quantitative detection of classical swine fever virus antibody based on double detection line technology, the method comprising: sample pad, classical swine fever virus E2 protein gold standard pad, rabbit IgG gold standard pad, water-absorbing pad , the nitrocellulose membrane coated with the double detection line of the classical swine fever virus E2a protein and the quality control line of goat anti-rabbit IgG, and the PVC rubber plate according to the image 3 Assembled sequentially to obtain the test paper card for quantitative detection of classical swine fever virus antibody.
[0070] Wherein, refer to Example 1 for the preparation method of CSFV E2 and E2a recombinant antigens.
[0071] Preparation method of classical swine fever virus E2 protein gold label pad: tak...
Embodiment 3
[0079] The preparation of embodiment 3 swine fever virus antibody quantitative detection test paper card
[0080] The embodiment of the present invention provides a preparation method of a test paper card for quantitative detection of classical swine fever virus antibody based on double detection line technology, the method comprising: sample pad, classical swine fever virus E2 protein gold standard pad, rabbit IgG gold standard pad, water-absorbing pad , the nitrocellulose membrane coated with the double detection line of the classical swine fever virus E2a protein and the quality control line of goat anti-rabbit IgG, and the PVC rubber plate according to the image 3 Assembled sequentially to obtain the test paper card for quantitative detection of classical swine fever virus antibody.
[0081] Wherein, the preparation method of the recombinant antigen of classical swine fever virus E2 and E2a refers to Example 2.
[0082] Preparation method of classical swine fever virus E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com