Acrylic resin bone cement capable of continuously and efficiently releasing antibiotics, and preparation method thereof
A technology of acrylic resin and bone cement, which is used in pharmaceutical formulations, medical science, prostheses, etc. to achieve obvious effects, bacteriostatic effects, and sustained and efficient release.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0016] The preparation method of the acrylic resin bone cement for sustained and high-efficiency release of antibiotics comprises the following steps:
[0017] (1) Preparation of SBA-15 carrier: 3-5g of P123 (polyethylene oxide-polypropylene oxide-polyethylene oxide triblock copolymer) dissolved in 20-50g water and 100-140g, 2M Add 6-10g of tetraethyl orthosilicate to the hydrochloric acid aqueous solution under stirring, react at 30-40°C for 20h, then stand at 80-120°C for 12h, and wash the obtained solid with water and absolute ethanol until medium SBA-15 is obtained by drying at 100°C for 10-20h and calcining in air at 500°C for 6-8h.
[0018] (2) Preparation of PEG-modified SBA-15 carrier: Dissolve 0-0.05g PEG in 5-10mL water, add 0.25-5g mesoporous molecular sieve SBA-15, stir evenly, heat to evaporate water, and vacuum dry.
[0019] (3) Preparation of drug-loaded filling materials: Dissolve 60-100 mg of gentamicin in 4-8 mL of water, add the PEG-modified SBA-15 carrier...
Embodiment 2
[0027] Preparation of SBA-15 carrier: 4g of P123 (polyethylene oxide-polypropylene oxide-polyethylene oxide triblock copolymer) was dissolved in 30g water and 120g, 2M hydrochloric acid aqueous solution, and then added under stirring 8g of tetraethyl orthosilicate, reacted at 35°C for 20h, then stood at 100°C for 12h, washed the obtained solid with water and absolute ethanol until neutral, dried at 100°C for 15h, and calcined in air at 500°C for 6h to obtain SBA-15.
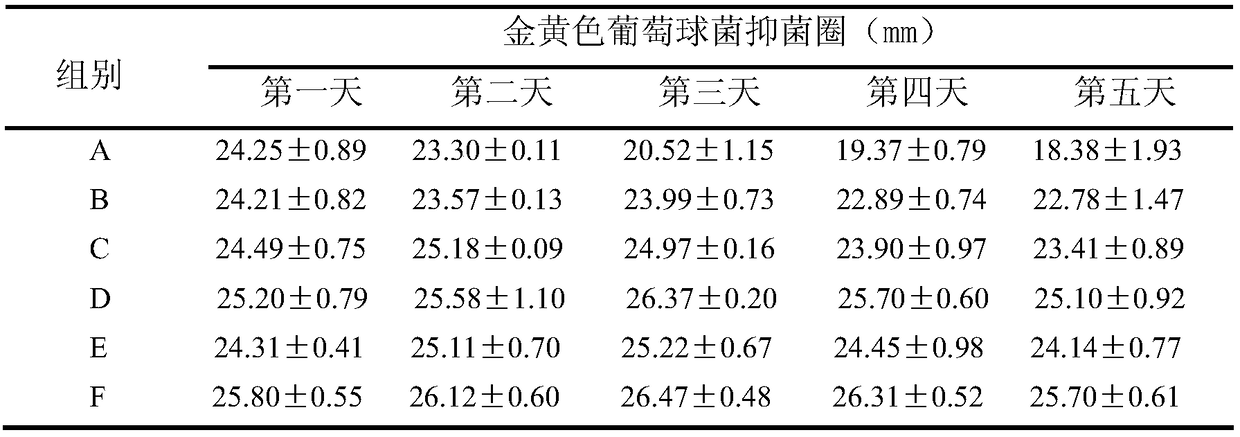
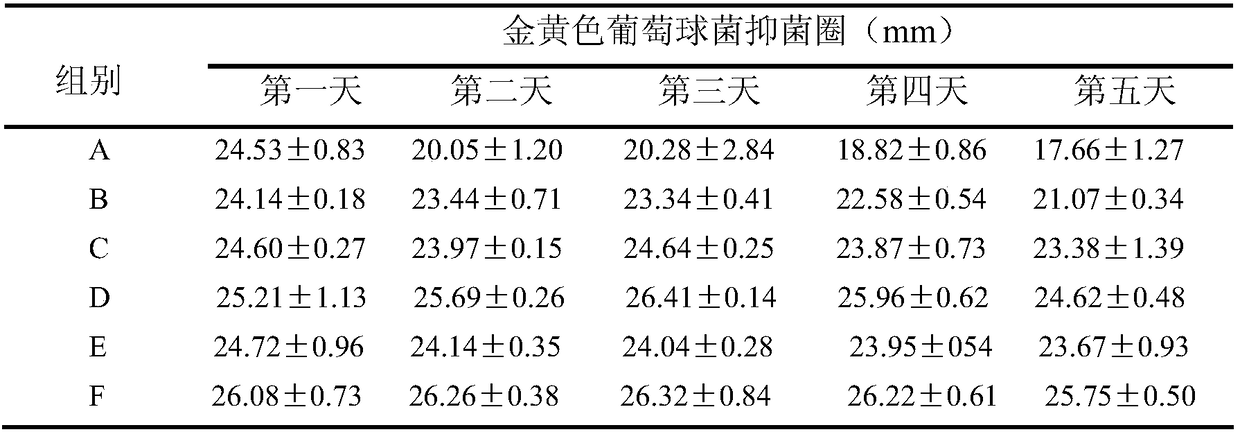
[0028] Preparation of the drug-loaded filling material: Dissolve 80 mg of gentamicin in 6 mL of water, add 0.25 g of SBA-15 carrier, stir for 24 hours, and dry at 80° C. for 12 hours to obtain the drug-loaded filling material.
[0029] Preparation of antibacterial bone cement: dope 10wt.% drug-loaded filling material in acrylic resin bone cement powder, mix and stir for 20 minutes to obtain solid phase bone cement powder, and then mix according to the needs of every 2g of solid phase mixture. The proportion of t...
Embodiment 3
[0033] Preparation of PEG-modified SBA-15 carrier: Dissolve 0.025g PEG in 5mL water, add 0.25g mesoporous molecular sieve SBA-15, stir evenly, heat to evaporate water, cool naturally and then vacuum dry.
[0034] Preparation of the drug-loaded filling material: Dissolve 80 mg of gentamicin in 6 mL of water, add the PEG-modified SBA-15 carrier prepared above, stir for 24 hours, and dry at 80° C. for 12 hours to obtain the drug-loaded filling material.
[0035] Preparation of antibacterial bone cement: dope 10wt.% drug-loaded filling material in acrylic resin bone cement powder, mix and stir for 20 minutes to obtain solid phase bone cement powder, and then mix according to the needs of every 2g of solid phase mixture. The ratio of the liquid phase of the bone cement is 1g, and the liquid phase monomer of the bone cement is added to the prepared bone cement solid phase mixture, stirred for 40 seconds to obtain a uniform slurry, and the antibacterial bone cement is obtained after c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com